International pharmacopeia stipulate a 14 day sterility test period and 28 day mycoplasma test period. ATMPs may have a shelf-life of less than 48 hours. Traditional methods cannot be used for release testing of ATMPs prior to treatment. The risk of administering potentially contaminated ATMPs to patients urges both industry and authority to find solutions for rapid release testing.
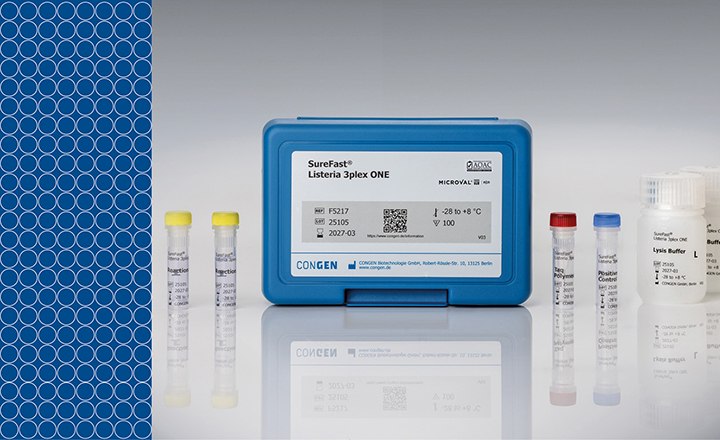
Sartorius cares for patient safety. After our long-term approved Mycoplasma Detection Kit, we recently released your next support: Microsart ® ATMP Bacteria
- total bacteria or mycoplasma detection within 3 hrs (Real-time PCR)
- qPCR Kit validation based on EP 5.1.6., EP 2.6.27, for the Bacteria detection kit and EP 2.6.7, EP 2.6.21 for the Mycoplasma detection kit
- Bacteria detection kit: Sensitivity proven for 12 selected bacterial species including 4 standard EP/USP strains
- Mycoplasma detection kit: Sensitivity proven for all of the 9 EP/USP mycoplasma species
- Lyophilized reagents - Stable reagents with constant high quality
- Specific TaqMan® probes reduce false-positives
- Non-infectious validation standards
- Less pipetting: controls already included
- All suitable qPCR cycler can be used
Stay tuned, the complementing Fungi detection kit will follow end of this year.
We are proud to support health.
Please note : Any products described on this page are
for Research Use Only and not intended for clinical diagnostic procedures unless otherwise stated.