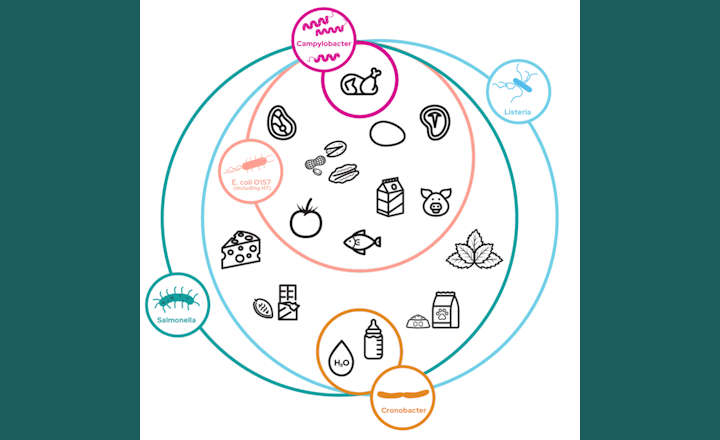
Achieving AOAC PTM status required a rigorous, independent laboratory examination of 3M’s unique molecular test method’s ability to accurately detect Listeria monocytogenes within a variety of foods.
Food samples analyzed during the validation study included beef hot dogs, queso fresco cheese, vanilla ice cream, 4 percent milk fat cottage cheese, 3 percent chocolate whole milk, romaine lettuce, bagged raw spinach, cold smoked salmon, deli turkey, raw chicken, cantaloupe, and various environmental surfaces (plastic, stainless steel, concrete).
Developed in response to customer engagement and ongoing desire to work with food processors to protect the world’s food supply, the latest Listeria monocytogenes assay is one of two test kits that were expanded on the innovative 3M™ Molecular Detection System platform.
The 3M Molecular Detection System is based on unique isothermal DNA amplification and bioluminescence detection technologies and designed around food processors’ needs for a real-time pathogen detection approach that’s faster and simpler while also more accurate.
The new test now provides a faster time-to-result – as little as 24 hours of enrichment – and features a streamlined workflow that is 30 percent faster than first generation assay.
For more information, visit www.3M.com/3MMolecularDetectionSystem/LMONOAOACPTM