Microbial monitoring in pharmaceutical and food production areas is a critical process focusing on detecting the presence of microorganisms in production surfaces, air or even personnel. This serves several important purposes:
- Product safety: By identifying potential contamination sources, it helps prevent microbial contamination of products.
- Quality control: Regular monitoring ensures that production environments meet the required specifications
- Trend analysis: Detecting changes over time allows for proactive measures to address emerging issues.
- Regulatory compliance: Many industries must maintain specified environmental standards such as EU GMP Annex 1, ISO 14644 standards, FDA guidelines, USP guidelines, and specific industry regulations like FSMA for food production.
- Process validation: Help verify that cleaning and sanitization procedures have been effective.
Typical monitoring targets include:
Surfaces - Work areas, Equipment, and other Contact Surfaces:
The ever-present biofilms can often pose significant problems affecting processing efficiency and product quality. Accurately identifying the presence of biofilms is crucial for taking effective remedial action.
Mariza Fraser, EIT International, highlights Bactiscan's effectiveness in biofilm detection across industries. In addition, we learn from Professor Sandra Cristino, Bologna University, whose research focuses on drinking water quality risks, how her team is using a new approach to estimating the presence of other bacteria that could support biofilm formation by combining water sampling with swabbing using Copan's Swab Rinse Kit (SRK®) to study water contamination at the point of use.
Medical Wire's POLYWIPE™ can get through the protective barrier of biofilm, ensuring thorough sample collection and together with NRS II™ provides a comprehensive solution to biofilm contamination and the challenges posed by residual cleaning chemicals.
Ideal for verification of sanitation protocols, Puritan's EnviroMax® and EnviroMax Plus® (premoistened) swabs have large, foam, paddle-like swabs for sampling larger areas. ESK® (Environmental Sampling Kit), includes a smaller swab for hard-to-reach spots and is available in four solution options, and two fill levels.
With identification results in just two minutes Microgen rapid latex agglutination tests, provide fast, easy, and cost-efficient methods for confirmatory identification of Salmonella, Legionella or Staphylococcus aureus colonies in food and environmental samples. DOWNLOAD Product Flyers.
Get your FREE SAMPLE of CondaLab's new Lock&Block contact plates, which have an innovative closing system that allows the plate lid to be secured and safely transported to the lab without risk of contamination. Their contact and RODAC Lateral Code (LC) plates have a convenient side label, making results reading more convenient.
Microbial Air Sampling and Monitoring
To eliminate the need for autoclaving aluminum lids for air samplers, Merck introduce the new Safe Lid for MAS-100 NT® Air Samplers, these are disposable, sterile, and ready-to-use perforated lids designed to optimize air sampling.
Cherwell provides both off-the-shelf and bespoke environmental monitoring solutions. Including Redipor® ready-to-use media, the next-generation BAMS biofluorescent particle counter offers real-time microbial detection and active air samplers, plus expert support for custom media formulation and calibration and servicing, ensuring cleanroom compliance and accuracy of results.
HiMedia's product range includes gamma-irradiated and triple-wrapped culture media plates, microbial air samplers, and swabs, ensuring comprehensive coverage for your monitoring needs.
Automation and Data Digitalisation
Dr. Steven Giglio, Clever Culture Systems discusses AI integration. The APAS Automated Plate Assessment System is a game changer for EM compliance in pharma manufacturing, ask for a FREE DEMO!
Standardise your process from sample collection to result with bioMérieux's 3P® CONNECT SOFTWARE, designed by and for pharmaceutical EM specialists, with easy integration into your IT environment. WATCH A VIDEO on how you can skip the paper-based process, decrease the risk of errors, and save time during the entire EM process.
For food manufacturers, bioMérieux's "Augmented Diagnostics" approach helps to integrate diverse data sources, breaking down silos and creating an optimized environmental monitoring program. WATCH A VIDEO on the Environmental Monitoring Program in Food Production - Augmented Diagnostics Approach.
Quality Control and Proficiency Testing
Discover from Microbiologics why you should include your facility's environmental isolates in the QC testing schedule. To ensure isolate controls are ready for reordering with quick turnaround times and low minimum quantities, Microbiologics preserves and stores all custom strains so that labs can maintain their QC programs without delays. GET A QUOTE!
LGC AXIO offers Proficiency Testing Schemes covering food, beverage, consumer safety, and water and environment sectors. The AXIO HYGIENE scheme for surface monitoring offers a comprehensive range of test materials and analytes, including total microbial counts and pathogens, as well as yeast and mould plus new for 2025, will be PT-HY-15 Detection of Cronobacter in Sponge, LGC AXIO Proficiency Testing Catalogue VIEW FLIPBOOK details all the new sample launches for 2025. The AIR scheme supports a laboratory’s quality system by providing an external and independent assessment of its performance in conducting testing of air and stacks emissions.
EM-Body Connectivity With 3P® CONNECT SOFTWARE
From planning to data reporting, pharmaceutical sites now have the ability to supervise EM control at all stages of the process thanks to 3P® CONNECT. The result: greater peace of mind thanks to standardized EM control and financial savings!
Find Out More
Safeguarding the Environment: Advanced Microbial Monitoring and Water Purification Techniques
In this interview with Professor Sandra Cristino, Bologna University, Italy, we have an opportunity to explore Professor Cristino’s innovative work in monitoring drinking water quality and the risk it can pose to public health.
Find Out More
Addressing Cleanroom Contamination Risks with Disposables
The Safe Lid for MAS-100 NT® Air Samplers is a disposable, sterile, and ready-to-use perforated lid designed to optimize air sampling. Made from 100% recyclable ABS, each box contains 30 sterile, individually double-bagged lids. This time-saving solution eliminates the need for autoclaving.
Find Out More
Condalab, Your Trusted Companion in Surface Control
Learn how Condalab’s innovative products improve surface control and ensure microbiological safety.
Find Out More
How AI is Changing Compliance in cGMP Environmental Monitoring
In this interview with Dr. Steven Giglio, Clever Culture Systems' Scientific Director, we explore how EM compliance meets cutting-edge AI tech. We'll discuss how this groundbreaking innovation reshapes compliance basics in pharmaceutical manufacturing.
Find Out More
Verify Your Sanitation Protocol with Puritan® Surface Sampling
Assure the safety of your products with Puritan surface sampling kits—for monitoring your sanitation protocols and to be sure your surfaces are free from contaminants—before production begins.
Find Out More
bioMérieux Transforms Environmental Monitoring – Move From Reaction to Prevention
Environmental monitoring is critical throughout the entire food production process, from planning and execution to problem-solving and optimization. By automating your environmental monitoring program with digital tools, bioMérieux helps you connect the dots in your facility.
Find Out More
Biofilm: The Hidden Menace - But Not for NRS II™ or POLYWIPE™!
POLYWIPE™ and NRS II™ remove biofilm and neutralise cleaning agents, ensuring accurate monitoring across various industries.
Find Out More
Uncover the Invisible Threat: Bactiscan's Precision Biofilm Detection
In this interview, we explore with Mariza Fraser, Global Sales Manager, EIT International, the remarkable capabilities of the innovative Bactiscan tool and discover why it's becoming the go-to solution for industries battling with biofilms.
Find Out More
The Case for Incorporating Environmental Isolates into Routine QC
Incorporating environmental isolates into quality control ensures pharmaceutical microbiology labs meet regulatory expectations and enhance contamination control. Local strains improve detection of microbial risks, safeguarding product quality and patient safety.
Find Out More
Check Out LGC AXIO Proficiency Testing's HYGIENE and AIR Schemes
Join LGC AXIO Proficiency Testing's global network of labs, participating in high-quality, flexible PT schemes for Hygiene Surface Monitoring (HYGIENE) and Air & Stacks Emissions (AIR).
Find Out More
Cherwell: Leading EM Solutions for Cleanroom Compliance
Cherwell offers superior environmental monitoring solutions, including Redipor® media and the cutting-edge BAMS unit. Its range and adaptability ensure effective contamination control and compliance in a variety of cleanroom environments.
Find Out More
Microbiological Culture Media and Air Samplers for EM of Air and Surfaces
Effective microbial monitoring is essential for maintaining control over manufacturing facilities, particularly when producing sterile medicinal products. Ensuring that environmental conditions meet stringent regulatory standards is critical for product safety and quality.
Find Out More
Microgen Latex Agglutination Kits for Salmonella, Legionella or Staphylococcus aureus ID
Get fast and reliable results confirmed in 2 minutes for confirmatory identification of Salmonella, Legionella or Staphylococcus aureus colonies in food and environmental samples.
Find Out More
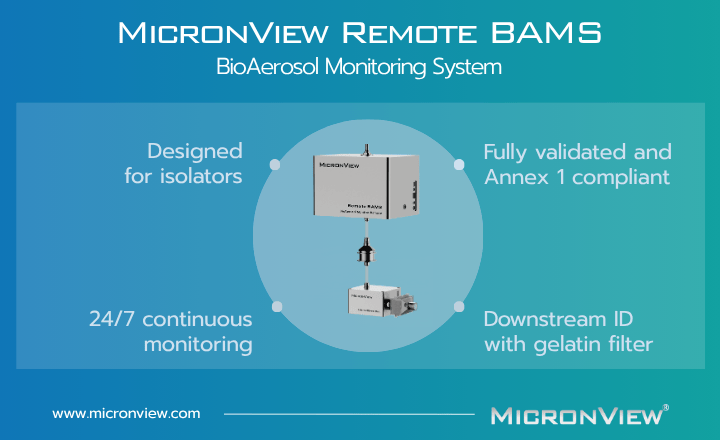
MicronView’s Remote Monitoring Solutions for Isolators
MicronView’s Remote BAMS, APC, and BAS are designed for use in isolators, offering viable and/or particle monitoring in a compact, easy-to-install package. Paired with advanced software, these units streamline contamination control and data analysis.
Find Out More
Suitability of a Single Incubation Temperature for EM Programs
Dual temperature incubation is currently among the most common EM practices. However, a well-defined single temperature period practice could replace the dual temperatures regime. Download our white paper to learn how you could improve productivity.
Find Out More