- Employs Next-Generation Solid-Phase Cytometry (SPC) to detect microorganisms in cell-based therapies within a day.
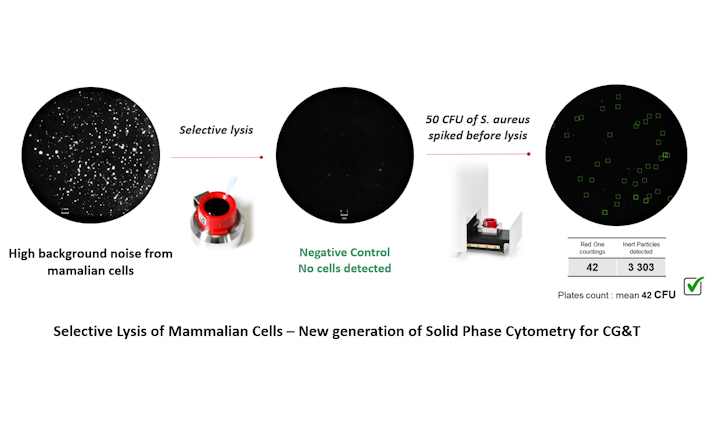
- Redberry developed a specific reagent that selectively lyses mammalian cells without harming the microorganisms.
- The technique has been validated on various cell types and complies with European Pharmacopoeia 2.6.27.
A major challenge in developing and testing Advanced Therapy Medicinal Products (ATMPs) is maintaining sterile conditions throughout manufacturing. Contamination poses significant health risks to patients, making reliable microbiological quality assessments essential before administration. Traditional methods can take up to 14 days—too long, considering the short shelf life of these products. Therefore, faster testing is crucial for ensuring the safety of these therapies.
Redberry has recently introduced a new technique to early adopters, employing Next-Generation Solid-Phase Cytometry (SPC) to detect microorganisms in cell-based therapies within a day. This method uses staining to identify microorganisms based on their metabolic activity. A significant challenge is the active metabolism of the mammalian cells in these therapies. Redberry developed a specific reagent that selectively lyses mammalian cells without harming the microorganisms to address this. This allows the technique to accurately and quickly differentiate between therapeutic cells and contaminants.
In collaboration with ATMP manufacturers, this technique's efficacy was validated on various cell types, including HeLa, Jurkat, Stem, CAR-T, and CHO cells. It complies with the European Pharmacopoeia 2.6.27, considering a detection limit of 100 CFU. The process requires an activation phase similar to that used in the commercialized Bioburden application. Watch the video here.
Additionally, it can reactivate anaerobic strains in less than six hours, establishing a new standard in rapid microbial testing.
This new application complements the 4-day sterility test introduced by Redberry last year and which is fully compatible with cell-based matrices. Click here for more information or use the Request Information button below to connect with the company.