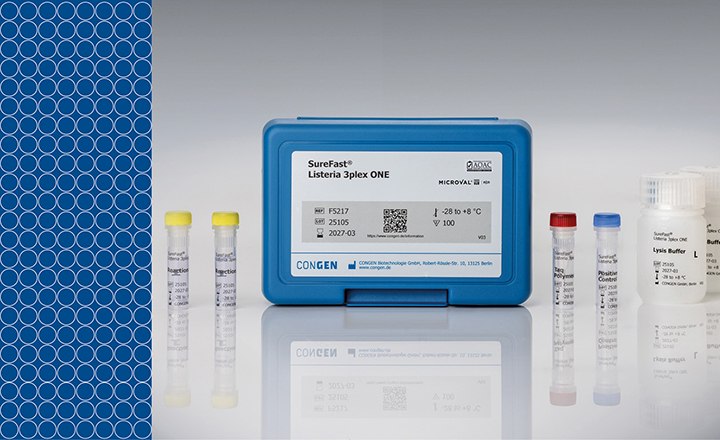
The Microsart® ATMP Mycoplasma real-time PCR kit is designed for all hospitals, institutions, and companies involved in testing for mycoplasma contamination in cell-based therapeutics and other advanced therapy medical products (ATMPs). The kit was validated according to EP 2.6.7 and EP 2.6.21 with respect to detection limits for all listed mycoplasma species, specificity, and robustness in autologous cell transplants (e.g. chondrocytes).
The Microsart® ATMP Mycoplasma kit utilizes quantitative, real-time PCR (qPCR) as the method of choice for sensitive and reliable detection of mycoplasma contamination and combines several technical features:
- Mycoplasma detection within 3 hours
- Comprehensive qPCR kit validation based on EP 2.6.7 for compliant final release testing
- Highest Specificity by TaqMan™ probes
- Two results in a single well: 1. Mycoplasma contamination result (FAM™ channel) and 2. PCR inhibition control (ROX™ channel) in one pipetting step (duplex-assay)
- Maximal reliability of the result with up to 75% less pipetting compared to single assays
- Non-infectious validation standards are already pre-quantified with 10 CFU/ml and ready-to-use for matrix-specific validation studies without any risk of cross-contamination
The Microsart® ATMP Mycoplasma kit is the optimal solution for all QC labs performing mycoplasma testing of cell-based therapeutics.
We are proud to support health.