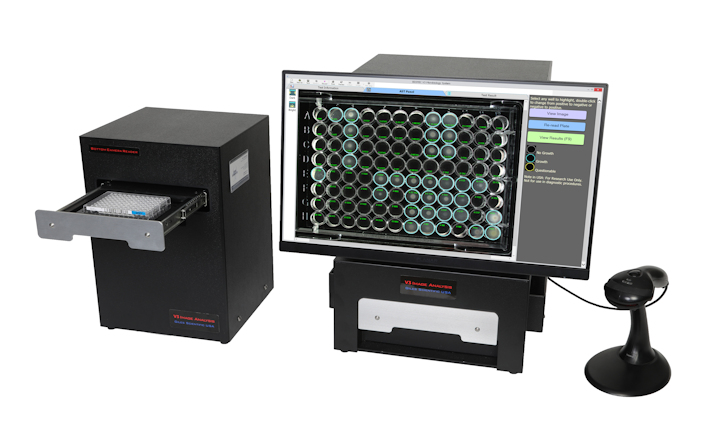
bioMérieux has announced FDA 510(k) clearance for VITEK® COMPACT PRO, enhancing microorganism identification (ID) and antibiotic susceptibility testing (AST).
This system aims to combat antimicrobial resistance (AMR) and improve diagnostic accuracy in clinical and industrial laboratories.
Annually, 11 million deaths occur from sepsis, with 1.3 million linked to antibiotic-resistant bacteria, underscoring the need for efficient diagnostics.
VITEK® COMPACT PRO offers:
- Ergonomic design and simplified workflow for improved user experience.
- Fastest routine ID/AST results, enhancing laboratory efficiency.
- Ideal for small and medium-sized labs transitioning from manual to automated processes.
The product will ensure the quality and safety of food, pharmaceutical, and cosmetic products in industrial settings.
The commercial launch is set for select countries, with a global rollout planned for the second quarter of 2025.
Read the full press release here.