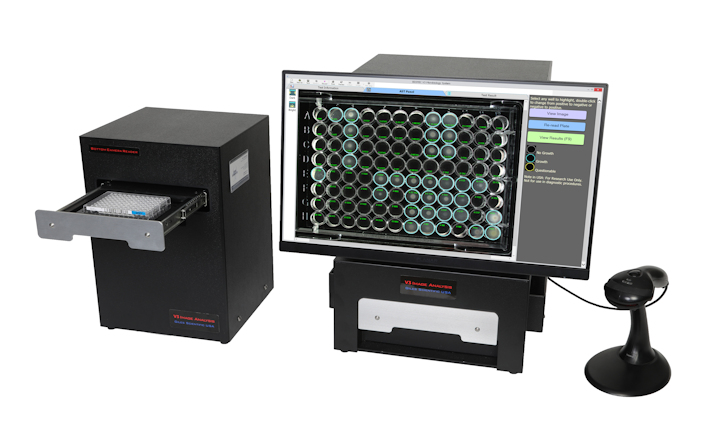
BIOMIC V3 is an imaging system that automates the reading and interpretation of clinical microbiology tests from various manufacturers. BIOMIC V3 will include the following CLSI and EUCAST guidelines in the next software update available in April/May 2025.
CLSI Guidelines:
- M100, 35th Edition: Performance Standards for Antimicrobial Susceptibility Testing (January 2025)
EUCAST Guidelines:
- Breakpoint Tables for Interpretation of MICs and Zone Diameters v 15.0 (January 2025)
- Routine and extended internal quality control for MIC determination and disk diffusion as recommended by EUCAST. v 15.0 (January 2025)
- Zone diameter breakpoints for rapid antimicrobial susceptibility testing (RAST) directly from blood culture bottles. v 7.2 (September 2024)
Please note : Any products described on this page are
for Research Use Only and not intended for clinical diagnostic procedures unless otherwise stated.