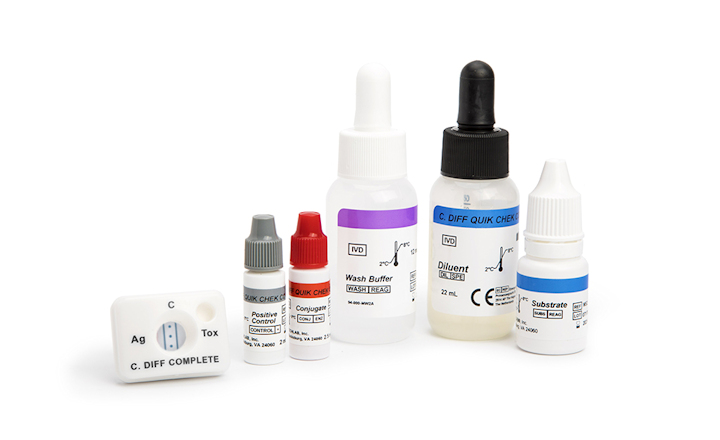
- Rapid membrane enzyme immunoassay for simultaneous detection of Clostridioides difficile GDH antigen and toxins A and B.
- Provides GDH and toxin results within 30 minutes.
The C. DIFF QUIK CHEK COMPLETE® test is a rapid membrane enzyme immunoassay designed for the simultaneous detection of Clostridioides difficile glutamate dehydrogenase (GDH) antigen and toxins A and B in a single reaction well. This innovative test provides both GDH and toxin results within approximately 30 minutes, enabling healthcare professionals to differentiate between active C. difficile infection and colonization in a cost-effective and clinically relevant manner.
"We are proud to achieve IVDR certification for our C. DIFF QUIK CHEK COMPLETE® test," said Christina Lindved, CEO of SSI Diagnostica Group, "This achievement underscores SSI Diagnostica’s commitment to delivering high-quality diagnostic tools that meet the stringent regulatory requirements and fulfill the needs of healthcare providers and patients worldwide."
This certification recognizes the test's adherence to rigorous quality and safety standards, ensuring its reliability and effectiveness across European clinical settings. It also marks a significant advancement in SSI Diagnostica’s efforts to enhance diagnostic capabilities for C. difficile infections.
For more information about the C. DIFF QUIK CHEK COMPLETE® test and the TECHLAB portfolio of diagnostic solutions, please visit www.techlab.com or use the Request Information button below to connect with the company.
To see the rest of the SSI Diagnostica Group portfolio, please visit www.ssidiagnostica.com.
* This assertion is based on a review of the EUDAMED database, 06/03/2024 at 10:00 am ET USA, which lists IVDR-compliant devices under code W0105011803.