The Green Revolution in Endotoxin Testing Reagents
In July 2024, the USP Microbiology Expert Committee approved the inclusion of Chapter <86> Bacterial Endotoxins Test Using Recombinant Reagents to the United States Pharmacopeia–National Formulary (USP–NF). This allows the use of non-animal-derived reagents for endotoxin testing; the final text of the chapter will become official in May 2025.
This introduction of sustainable reagents for endotoxin testing reflects an increasing awareness of the environmental impact of laboratory reagents. By embracing more eco-friendly alternatives, the industry is taking a significant step towards reducing its environmental impact without compromising on quality or efficacy.
However, the shift towards sustainable reagents brings some challenges for regulatory compliance:
- Adaptation of Testing Protocols: Existing procedures may need to be revised to incorporate these new, sustainable reagents.
- Validation Requirements: Companies will likely need to re-validate their methods to ensure compliance with the updated standards.
- Supply Chain Considerations: Sourcing and integrating sustainable reagents may require adjustments to procurement strategies.
The bacterial endotoxins tests described in the new chapter are additional techniques to the current tests described in Chapter <85> Bacterial Endotoxins Test (BET). The new chapter includes methods that use both recombinant cascade (rCR) and recombinant Factor C (rFC) reagents and provides information for manufacturers of new and existing pharmaceutical products on how to incorporate them into their quality testing.
These recombinant technologies offer a game-changing advantage: they eliminate the need for animal-derived products, aligning perfectly with corporate global initiatives to create more sustainable and ethical testing methods. But that's not all - by adopting these techniques, manufacturers can fortify their supply chains against potential interruptions, ensuring more reliable and consistent testing processes.
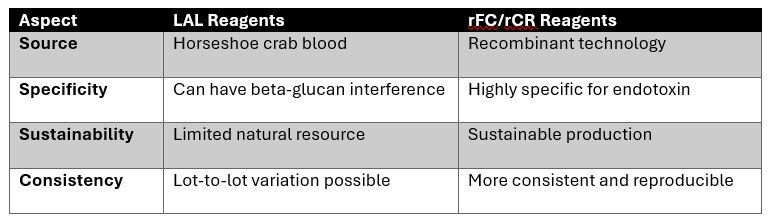
Key differences between LAL and rFC/rCR
Here, we've invited experts in the field of recombinant endotoxin reagents to discuss the unique features and benefits of their company's solutions, and how to align your lab practices with these updated regulations to ensure regulatory compliance and a more sustainable testing regimen for your facility.
Discover from Allen Burgenson, Global SME in Bioprocessing Testing at Lonza, how implementing Lonza's rFC assay can allow companies to enhance their product safety measures while optimizing their testing processes in the critical field of endotoxin detection. Allen includes a handy reference - BET Assay Reaction Comparison chart showing the signal amplification steps and measured characteristics for each of Gel Clot, Kinetic Turbidimetric, Endpoint Chromogenic, Kinetic Chromogenic and rFC assays.
Timothy Francis and Delaney Novak, Technical Specialists in the LAL Division of FUJIFILM Irvine Scientific, provide their expert perspectives on how FUJIFILM Wako's rCR PYROSTAR™ Neo+ may help increase the accuracy and sustainability of endotoxin testing. They also describe a key advantage of using rCR PYROSTAR™ Neo+ is that it uses the same methodology, equipment, and accessories as natural LAL does.
Get a unique chance for a Question and Answer session with Courtney Wachtel, Product Manager, Charles River, who highlights how using a self-contained, cartridge approach (available with either LAL or rFC reagents) offers a highly controlled environment for reactions, reducing analyst variability and potential contamination risks.
bioMérieux offers a downloadable summary document to learn more about the implications of the new USP Chapter <86> for endotoxin testing.
Are you ready to elevate your endotoxin testing to the next level?
A Q&A on USP, Recombinant Innovation, and Future Trends
USP supports recombinant methods as alternatives to LAL. Courtney Wachtel of Charles River explains the regulatory shift, the benefits of recombinant and cartridge-based testing, and how labs can enhance efficiency, sustainability, and compliance.
Find Out More
Sustainable and Compendial Endotoxin Testing with Recombinant PYROSTAR™ Neo+
Timothy Francis and Delaney Novak, Technical Specialists in the LAL Division of FUJIFILM Irvine Scientific, share their expert insights into how utilizing FUJIFILM Wako's rCR PYROSTAR™ Neo+ can improve both the accuracy and sustainability of endotoxin testing.
Find Out More
Lonza’s rFC: Sensitivity That Sets the Standard
Allen Burgenson, Global SME in Bioprocessing Testing at Lonza, explains why Lonza’s PyroGene® recombinant Factor C assay's cutting-edge technology has the power to transform endotoxin testing workflows, providing researchers and manufacturers with the confidence to push the boundaries of innovation.
Find Out More
USP Chapter <86> and the Shift to Recombinant Methods
What does USP <86> mean for you? Explore the implications of the new USP Chapter <86> for endotoxin testing and the ground-breaking developments in synthetic Bacterial Endotoxin Test methods. Download the Scientific Summary here.
Find Out More
2025 Endotoxin Testing Summit
Join industry leaders for a two-day summit on sustainable endotoxin testing. Explore innovations like recombinant Factor C & MAT. Reserve your spot today!
Find Out More
Find Out More
Find Out More