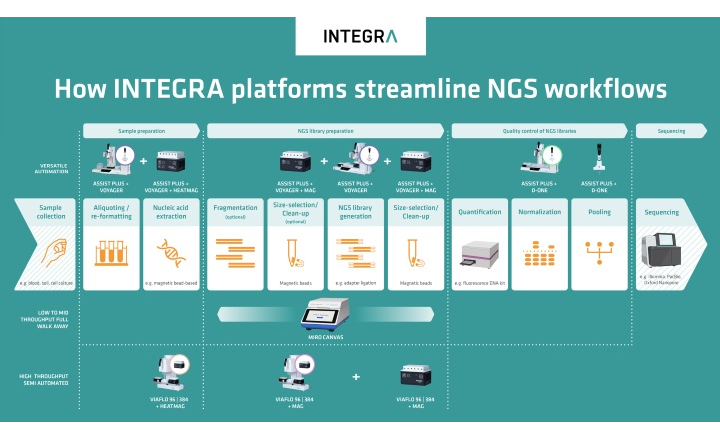
Charles River is pleased to introduce the first fully automated robotic system developed specifically for high-volume endotoxin testing in the central QC lab: the Endosafe® Nexus™.
Ideal for high-volume water testing or samples that require dilutions, the Nexus™ tests 48 to 60 samples per run with minimal preparation and supervision. The system integrates limulus amebocyte lysate (LAL) cartridge technology, precise liquid handling and data management into one automated platform. Users need only place bar-coded samples on the Nexus™ deck. Samples are scanned, diluted with the proper diluent (if needed), and run on EndoScan-V™ software. Results can be printed and sent directly to the laboratory information management system (LIMS).
The efficiency of the Endosafe® Nexus™ is evident not only in its testing capacity, but also in its design. Precise LAL cartridge technology reduces test variability and the need for subsequent investigations. Utilizing bar codes to store product information, the Nexus™ eliminates the need for a robotic script expert on staff and frees up lab personnel to focus on value-added tasks. Likewise, the streamlined design of the Nexus™ minimizes its lab footprint, freeing up space for other equipment.
Click here to learn more about how the Endosafe® Nexus™ can help streamline processes in your lab.