Lonza's groundbreaking rFC (Recombinant Factor C) assay is transforming endotoxin testing in the bioprocessing industry. This innovative alternative to traditional LAL assays offers a reliable and efficient method for detecting bacterial endotoxins, which are essential to help ensure product safety. According to Allen Burgenson, Global SME in Bioprocessing Testing at Lonza, implementing Lonza's rFC assay allows companies to enhance their product safety measures while optimizing their testing processes in the critical field of endotoxin detection.
Q: How does the Lonza rFC assay compare to Lonza’s LAL offering?
Allen: Lonza’s PyroGene® recombinant Factor C assay was introduced in 2003 as a sustainable alternative to using Limulus Amebocyte Lysate, which is derived from the blood of the American Horseshoe Crab (Limulus polyphemus). The assay has the same sensitivity as our Kinetic QCL® LAL assay (0.005 EU/mL), and is less susceptible to interferences, including enhancement due to the presence of beta-glucans. Side-by-side comparisons show comparable results in most applications.
Q: How does the PyroGene® rFc Assay work? What applications is it most suitable for?
Allen: The PyroGene® rFC assay uses a recombinant form of Factor C that is found in every other endotoxin detection reagent (see Figure 1 below). It directly cleaves a fluorogenic substrate to yield light upon excitation, eliminating the need for the signal amplification steps found in classical assays which are subject to interferences.
Figure 1 - BET Assay Reaction Comparison
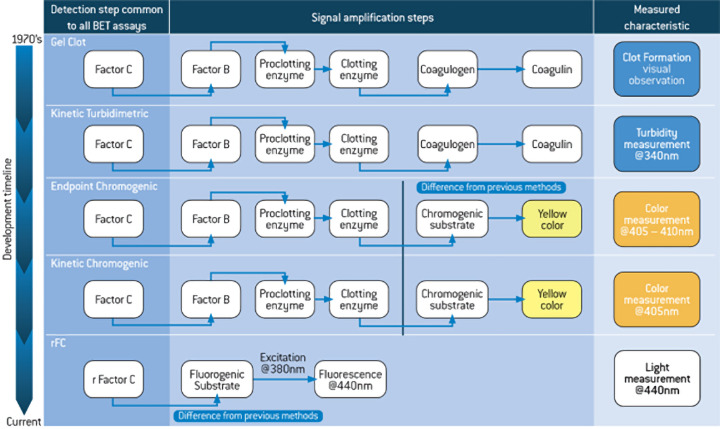
The PyroGene® rFC assay works in almost every application that our classical LAL reagents are used in, with the exception of products that are fluorescent. Most current applications are for water, which accounts for approximately 75-80% of all pharmaceutical testing.
Q: What are the most common problems labs face with LAL-based testing?
Allen: All LAL-based assays are subject to “enhancement” or a reaction making the apparent endotoxin level appear higher than it actually is. Such interference must be overcome using a modified assay or a substance like β-glucan blockers that block the Factor G pathway contained in LAL reagents. The PyroGene® rFC assay doesn’t follow the Factor G pathway.
Q: What makes Lonza an advanced solution when compared to other options in the market?
Allen: The PyroGene® rFC assay is a liquid preparation and as such, needs no reconstitution step. You simply mix the volume of components you need to test your samples, greatly reducing wasted reagents. This is ideal in high-volume or automated laboratories.
Q: Is the PyroGene® rFC assay suitable for automation?
Allen: Absolutely! The PyroGene® rFC assay has been used on the PyroTec® PRO robotic system in dozens of facilities around the world. Some are high-throughput facilities testing thousands of samples in a month, and the ease of preparing large volumes of reagent as needed is a significant benefit to them.
Q: How can Lonza support with documentation for audits?
Allen: Our dedicated teams can provide documentation to support customer audits and regulatory filings. Additionally, the method validation was published in the Jan/Feb 2010 issue of Pharmacopeial Forum, the official journal of the United States Pharmacopeia. A reprint of the article is also available upon request.
Q: What level of support can Lonza offer?
Allen: Lonza’s Scientific Support team and Global SME group are ready to assist customers with a wide range of applications, including testing, validation support, and regulatory filings. We also offer hands-on assay training, either at our facility or yours.
Q: Can you give any effective strategies on how to transition to rFC methods?
Allen: Yes, Lonza can provide a step-by-step validation protocol for our PyroGene® rFC assay. In addition, our Scientific Support, Services, and Global SME teams can provide support for product validation, comparability, and more.
Are you ready to revolutionize your endotoxin testing process? Experience the Lonza difference for yourself. Visit Lonza.com/PyroGene or use the green "Request Information" button below for more details.
About Allen Burgenson - Global Subject Matter Expert - Testing Solutions at Lonza Walkersville, Inc.