Prevention is the cornerstone of any contamination control strategy (CCS). This can incorporate strict hygiene procedures, control of incoming raw materials and packaging, and facility design. However, it is also key to identify as early as possible in the process when the potential for contamination has occurred. Environmental monitoring in the facility plays a key role here, and any detection results in corrective action being taken - the earlier, the better in terms of product loss.
EU Annex 1 specifically describes pharmaceutical manufacturing CCS. To maintain product quality, these procedures discover, assess, and control contamination threats.
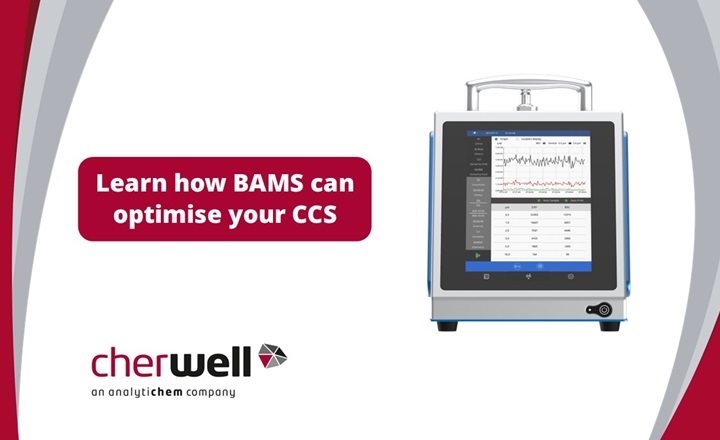
Cherwell, now part of AnalytiChem are renowned experts in the field of microbial air monitoring, having supplied and supported the SAS range for over 40 years. New technological innovations have now made real-time monitoring of bioaerosols possible. Cherwell is now able to offer real-time bioaerosol monitoring using the BioAerosol Monitoring System (BAMS). Here, we ask Christine Troutman, Senior Applications Scientist at MicronView, to share their in-depth knowledge and experience of air monitoring and how the BAMS can help improve a CCS, meet Annex 1 requirements, and hence reduce lost production.
Q: How does the BioAerosol Monitoring System (BAMS) work?
Christine: BAMS is a type of Biofluorescent Particle Counter (BFPC). BFPCs determine the biologic status and size of particles in air samples using fluorescence and light scattering. Particles are drawn into the instrument and scanned by a laser beam, which causes the light to scatter around the particle. This scattered light pattern is read by the instrument, and the size of the particle is determined.
Simultaneously, if the particles are viable, they will have metabolites (such as NADPH and Riboflavin) that are excited by the laser, causing them to emit fluorescence. This fluorescent signal is then detected and analyzed by the instrument. By analyzing the fluorescence and scattered light, the instrument can differentiate between biological and non-biological particles, providing real-time monitoring of microbial contamination in environments like pharmaceutical facilities and cleanrooms.
Q: What advantages does it have over conventional air sampling instruments?
Christine: BAMS offers several advantages over conventional growth-based contamination control strategies. Firstly, BAMS provides instantaneous results compared to the days required for traditional culture methods to produce visible colonies. This rapid detection allows for immediate corrective actions, minimizing the risk of product loss or contamination. This saves not only a significant amount of time but also production or product loss costs. Additionally, BAMS offers higher sensitivity, capable of detecting low levels of microbial contamination that may be missed by culture-based methods, as well as the ability to detect viable but not culturable (VNBC) organisms. This sensitivity is crucial in environments where even small microbial counts can pose a significant risk, such as in pharmaceutical manufacturing.
Furthermore, BAMS automates the counting and sizing of particles, reducing the potential for human error and increasing the reproducibility of results. BAMS also does not require any reagents or sample preparation, thus saving cost, time and increasing sustainability. Overall, BAMS provide a faster, more sensitive, and automated approach to contamination control, enhancing the efficiency and reliability of microbial detection in critical environments.
Q: Do the results need confirmation? How is that done?
Christine: Once BAMS is validated at the customer site, which involves a process of comparing BAMS to the current air sampling procedure, results obtained by the device are considered accurate. According to Annex 1, any microbial contamination should be identified if possible, so secondary air sampling with a traditional growth-based method can be conducted if an alert or action level is reached on BAMS. This allows the user to confirm the presence of contaminating microbes and attempt to identify the contaminating species. However, the majority of microorganisms present in the environment may not be culturable, so BAMS may detect the presence of organisms that the traditional air sampling device cannot.
Q: Which regulatory requirements can the BAMS help comply with?
Christine: There are many regulatory guidelines that BAMS can help users comply with, including but not limited to EU GMP Annex 1, ICH Q9 – Quality Risk Management, FDA Guidance – Sterile Drug Products Produces by Aseptic Processing, USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments, and ISO 14698 – Cleanrooms and Associated Controlled Environments.
BAMS offers significant benefits for compliance with the new regulations outlined in Annex 1. It enables real-time environmental monitoring, providing immediate feedback on airborne microbial contamination levels, a new requirement of Annex 1. In Grade A cleanroom environments, BAMS can be used as a complementary environmental monitoring tool in the detection of viable particles where 0 CFU counts are permitted. In all other cleanroom environments, BAMS also provides particle sizing and counting capabilities, aiding in risk assessment by identifying different types and sizes of particles that may carry microorganisms.
This rapid detection of contamination allows for prompt corrective actions to prevent product contamination, aligning with the requirements outlined in Annex 1 and the associated Contamination Control Strategy (CCS). Furthermore, BAMS complements traditional monitoring methods by offering continuous, digital monitoring data for improved data integrity and traceability, essential for regulatory compliance and audit purposes.
Q: What maintenance and calibration are needed?
Christine: BAMS requires very minimal maintenance if operated correctly. The exterior of the unit can be cleaned with commonly used disinfectants in the cleanroom environment. A yearly calibration is required to ensure both fluorescence detection and particle sizing abilities are functioning accurately.
Q: How do facilities start integrating the BAMS into their CCS? What validations are needed? How can Cherwell help?
Christine: Integrating BAMS into a new or existing Contamination Control Strategy (CCS) requires a thorough but straightforward validation process at the user site. This involves conducting a comparison study between the BAMS and existing air sampling technology to validate its functionality and establish alert and action levels. Additionally, a risk assessment study should be conducted to ensure that the BAMS is deployed in appropriate zones based on contamination risk levels. Cherwell offers assistance in formulating the validation plan and formalising the BAMS Standard Operating Procedure (SOP) based on user-specific CCS requirements.
Q: There are several real-time bioaerosol monitors on the market; what advantages does the BAMS offer?
Christine: BAMS offers some key advantages over other Biofluorescent Particle Counters (BFPCs) on the market. First, BAMS is highly portable, weighing only 15lbs/7kgs and having a battery life of approximately 6 hours. The small size of BAMS (255H X 200W X 264D mm) makes it extremely easy to integrate into current cleanroom systems. The portability of BAMS also makes it easy to conduct root cause investigations. Furthermore, BAMS also has an advanced software algorithm that serves to reduce false positive biological counts by reducing interferent signals. This is highly beneficial in cleanroom environments where even low counts may require an investigation. Finally, BAMS has an 8” touchscreen that can be used with two layers of gloves, allowing users to view results in real time and edit sampling plans with the simple and streamlined user interface.
Q: Can you share a user experience where the BAMS has been implemented and a clear ROI has resulted?
Christine: One significant benefit of using BAMS is during root cause investigations, which can lead to a clear return on investment (ROI). Unlike traditional growth-based sampling methods that take days to process and identify contamination, BAMS provides rapid results, facilitating quick discovery of the contamination source. This capability is particularly crucial as delays in identifying the cause could result in product loss. For instance, Pfizer conducted a case study in 2018 at a site in Puerto Rico following Hurricane Maria, aiming to return to Good Manufacturing Practice (GMP) conditions quickly and accurately. During this study, BAMS was used to scan HEPA filters and detect areas with increased biologic or particulate counts, indicating filter failure. This proactive approach to filter inspection helped Pfizer identify and replace at-risk filters promptly, minimizing delays and retests. The study demonstrated how BAMS can save time and resources, enhancing the process of returning to GMP conditions after an unplanned shutdown.
Learn more about the BioAerosol Monitoring System (BAMS) here.
About Christine Troutman