ALOA® One Day (Alternative Method) is a bioMérieux Gold Standard Method for Listeria spp. and Listeria monocytogenes: “One bag—One plate” with a time to result (TTR) of 44 hours.
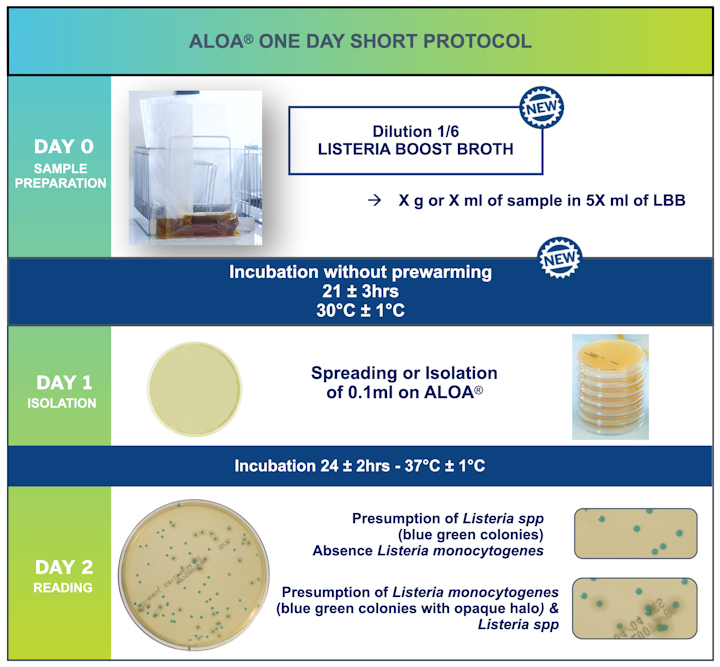
This NEW ALOA® ONE DAY short protocol provides reliable results with an enrichment time of 18 hours and good performance, plus the added benefits of a reduced environmental impact and cost savings for laboratories.
The new ALOA® ONE DAY short protocol allows for a significant reduction in volumes needed for routine analysis and a significant saving in terms of the costs associated with waste management.

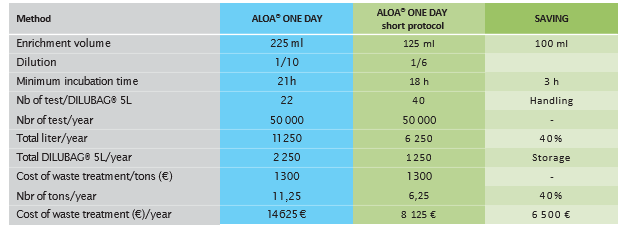
Example of savings for a 50,000 tests/year scenario. Estimated cost savings in volume thanks to the new ALOA® ONE DAY short protocol for 50,000 tests/year.
The new ALOA® ONE DAY short protocol method for Listeria monocytogenes detection in a broad range of foods and environmental samples showed equivalent results to the reference method with:
- A shorter broth incubation time (18h to 24h) allowing a faster time to result compared to reference and ALOA® ONE DAY methods
- A 40% reduction in culture media broth volume needed, leading to a 40 % decrease in waste treatment for laboratories
- Reduction Dilution: 1/10 with Half Fraser to 1/6 with Listeria Boost Broth
- Optimisation of sample preparation and laboratory workflows
- No prewarming step; storage at room temperature
- Enrichment step 18 – 24 Hours vs 22 – 27 Hours
- A reduction of 40% in incubation needed capacity for the enrichment step
- Less handling and storage - More samples in 1 Dilubag (40 samples instead of 22) + Reduction of 40% for reagents storage
- ISO 16140-2: Extension validation with the same full scope as ALOA® ONE DAY
Learn more or use the Request Information button below to contact bioMérieux.