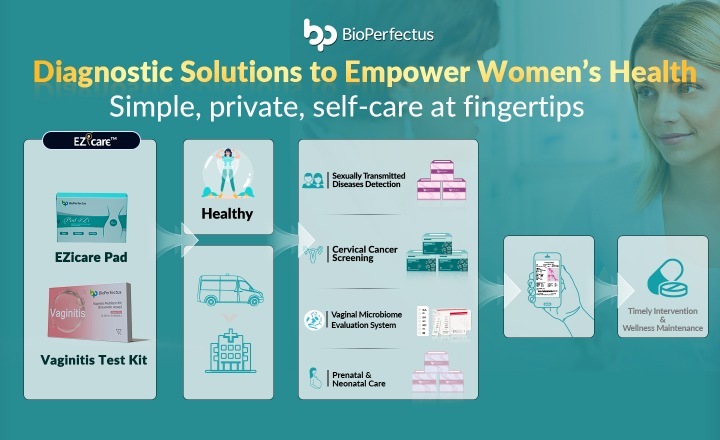
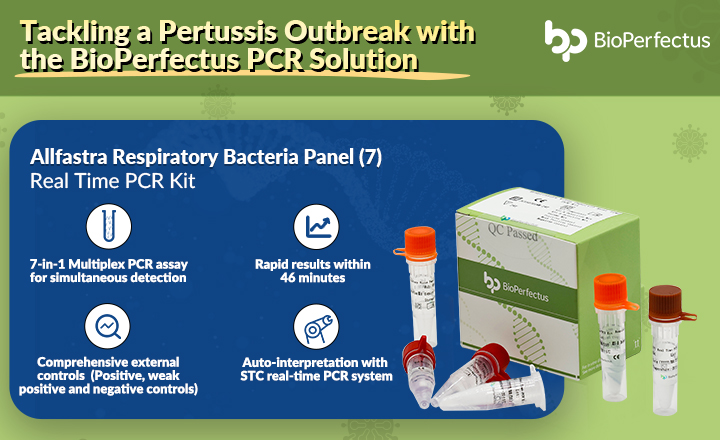
COVID-19 Coronavirus Real-Time PCR Kit is an In Vitro Diagnostic (IVD) reagent applying fluorescent PCR technology and aiming to qualitatively detect Open Reading Frame gene region (ORF1a/b) and viral nucleocapsid region (N) of SARS-CoV-2 RNA from upper and lower respiratory tract specimens.
Suitability: Upper respiratory tract specimens include throat swab and nasopharyngeal swab. Lower respiratory tract specimens include sputum. The product is intended for use on populations suspected to have SARS-CoV-2 infection as an aid in the diagnosis of SARS-CoV-2 infection by trained laboratory personnel on RT-PCR.
Key Points:
- Target region: ORF1a/b and N genes
- Clinical Sensitivity: 94.9%
- Clinical Specificity: 98.7%
- Limit of detection: 350 copies/mL
- Sample input volume: 5μL
- Sample type: Nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, mid-turbinate nasal swabs, nasal aspirates, nasal washes, bronchoalveolar lavage (BAL) fluid and sputum specimens.