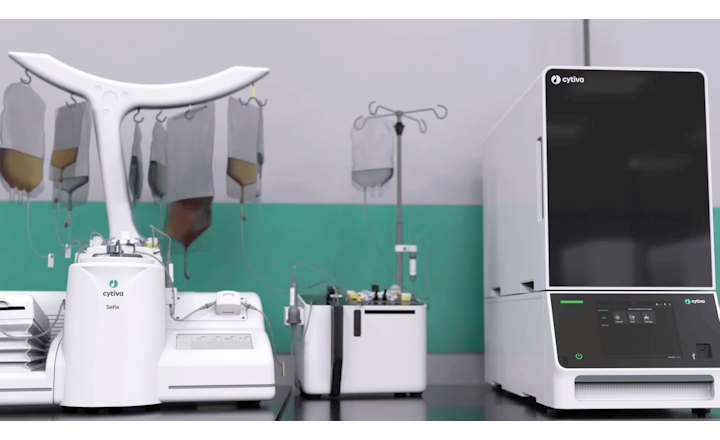
- Sefia cell therapy manufacturing platform comes out of Cytiva's extensive R&D expertise and in-depth understanding of CAR T manufacturing
- Automation features can accelerate productivity for transformative CAR T cell therapies at commercial scale
- The flexible and modular manufacturing platform is designed to minimize variability, increase efficiencies, and accelerate speed to clinical milestones
Cytiva is the tackling industry challenges that hinder patient access and wider adoption of autologous CAR T cell therapies with the new Sefia cell therapy manufacturing platform. As the latest innovation in the complete cell therapy workflow, this solution builds on Cytiva’s vast experience in manufacturing advanced therapies. The Sefia platform was developed as part of a collaboration with Kite, a Gilead Company, a global leader in autologous CAR T cell therapies.
Due to the personalized nature of autologous CAR T cell therapies, manufacturing practices have been heavily dependent on manual labor. They need to maintain product integrity in high-grade cleanrooms with skilled personnel, part of a complex manufacturing process. Limited capacity and the potential risk of batch failures have also made it difficult for drug developers to keep up with the growing patient demand. Cytiva’s Sefia cell therapy manufacturing platform has been designed with these specific challenges in mind.
Emmanuel Abate, President, Genomic Medicine, Cytiva, says: “Our new manufacturing platform is the culmination of more than a decade of scientific research. Working hand-in-hand with industry leaders, we developed a platform designed to significantly increase productivity, helping ensure patients can have greater access to life-changing advanced therapies. It’s also a reflection of both Kite and Cytiva’s commitment to collaboration and accelerating the manufacture of autologous CAR T cell therapies.”
At present, there are 10 autologous CAR-T cell therapies that are officially approved for use in the United States, the European Union, and Asia-Pacific. There are also more than 1000 cell therapy clinical trials underway globally, making flexible, scalable, and efficient manufacturing critical to meeting patient demand.
How it works
The Sefia cell therapy manufacturing platform unites the Sefia Select system and the Sefia expansion system. Using the modular approach and two functionally closed and digitally integrated systems, key steps in the workflow are automated. Sefia Select system automates the cell isolation, harvest, and formulation steps, while Sefia expansion system automates the cell activation, transduction, and cell expansion steps.
Automation eliminates many manual steps where human mistakes can add to manufacturing time and potentially cause cell stress. The platform can also be connected to Cytiva’s Chronicle automation software to monitor facility manufacturing operations and manage supply chain logistics.
Abate says: “Our goal has always been to help our customers scale-up the manufacture of these therapies while reducing risk factors. The Sefia platform was designed to help increase manufactured doses by up to 50% per year as compared to industry standard, which can help ultimately reduce the cost burden.”
Services provided by Cytiva
Cytiva customers benefit from its best-in-class service offerings and dedicated teams for everything from basic repairs to dedicated training programs for users, operators, and staff. Cytiva can also assist with quality documentation to ensure regulatory compliance at every step of the manufacturing workflow.