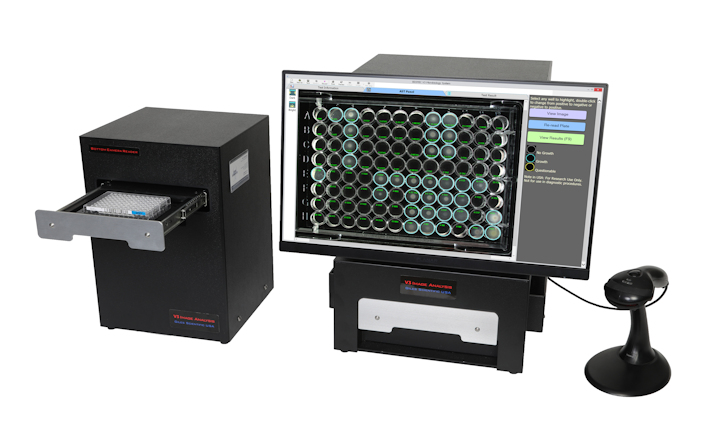
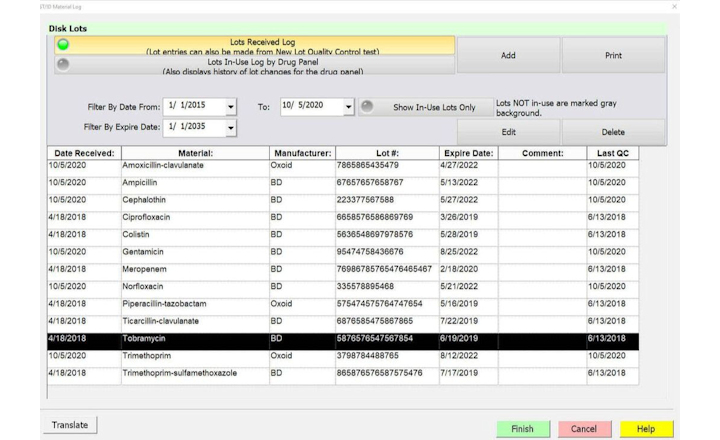
Charles River has prepared a poster presentation illustrating how Biotracker LIMS enables regulatory compliance thus safeguarding patient safety.
A custom, fully compliant LIMS that manages equipment, reagents, samples, workflow, and personnel, Biotracker offers the following QC laboratory modules to electronically capture data and comply with cGMPs regulation:- Audit trail
- Electronic signature
- Customer and Supplier management
- Configuration roles and security
- Sample chain of custody
- Workflow management
- Equipment/inventory control
- Report release
- CAPA/Deviation management
- Discrepancy management
Download the full poster here or contact the Supplier for more information using the green "Request Information" button below.