Liofilchem has received clearance from the FDA to market in the United States the Ampicillin*-Sulbactam (2/1) 0.016-256* MTS™ (MIC Test Strip), a quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of Ampicillin-sulbactam.
Ampicillin-sulbactam has been shown to be active both clinically and in vitro against the Gram-negative bacteria E. asburiae, E. cloacae, E. coli, K. aerogenes, K. oxytoca, K. pneumoniae, P. mirabilis and A. baumannii/A. calcoaceticus complex.
More technical details on the Ampicillin-Sulbactam MIC Test Strip IFU
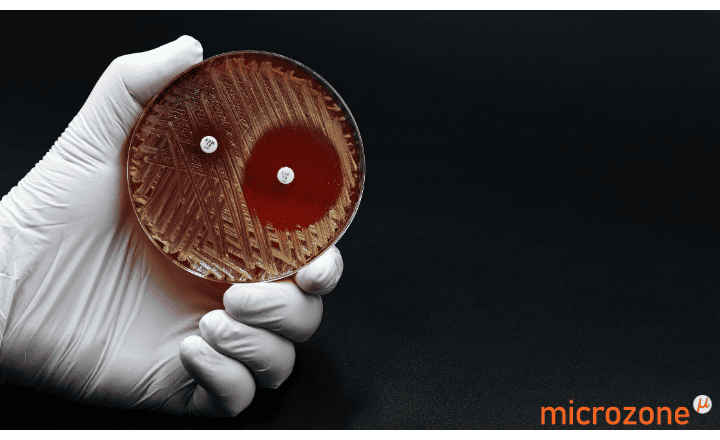
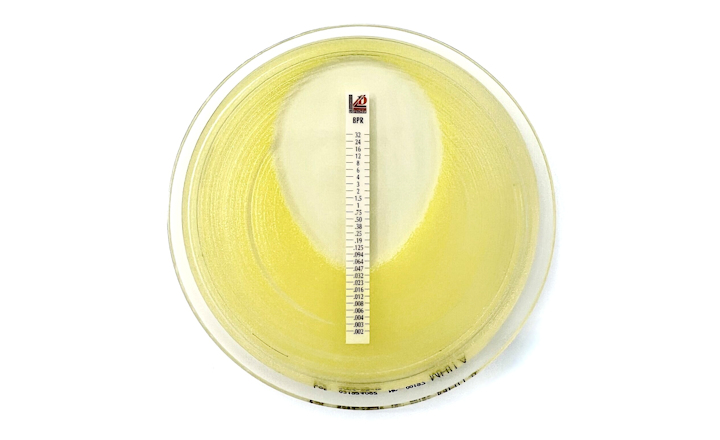
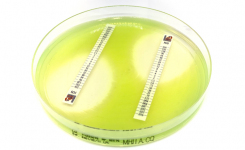
MTS™ (MIC Test Strips) are porous strips with special features (International Patent) that are impregnated with a predefined concentration gradient of antibiotic, across 15 two-fold dilutions of a conventional MIC method. On one side of the strip there is a printed MIC scale in μg/mL and a code that identifies the antimicrobial agent.
MTS™ Ampicillin*-sulbactam (2/1) 0.016-256*
- 10 strip pack: ref. 920271
- 30 strip pack: ref. 92027
- 100 strip pack: ref. 920270
The MTS™ range comprises antibiotics, antifungals, antimycobacterials, and combined strips for detection of resistance mechanisms (ESBL, MBL, KPC, AmpC and GRD) for over 150 items, each available in three pack formats (10, 30 and 100), for the widest gradient strip range in the international market.
Availability in USA:
Ampicillin-sulbactam is the most recent FDA approved item in the MTS™ (MIC Test Strip) product range, following approvals of Vancomycin, Dalbavancin, Ceftolozane-tazobactam, Meropenem, Ceftazidime, Telavancin, Tedizolid, Delafloxacin, Clindamycin, Erythromycin, Linezolid, Meropenem-vaborbactam, Azithromycin, Ceftazidime-avibactam, Plazomicin, Penicillin G , Eravacycline, Omadacycline, Tetracycline, Levofloxacin, Ciprofloxacin, Gentamicin, Doxycyclin, Imipenem-relebactm and Imipenem strips. The rest of the MTS™ range is available in the United States as research use only devices.
Availability in Europe and rest of the world:
The entire MTS™ product catalog is CE marked and fully available as IVD for clinical diagnostics purposes in Europe.
The MTS™ range is also compliant to MDSAP (Australia TGA, Brazil ANVISA, Health Canada, USA FDA, Japan MHLW/PMDA). It is registered at the competent Authority in many countries outside Europe as a clinical diagnostic device.