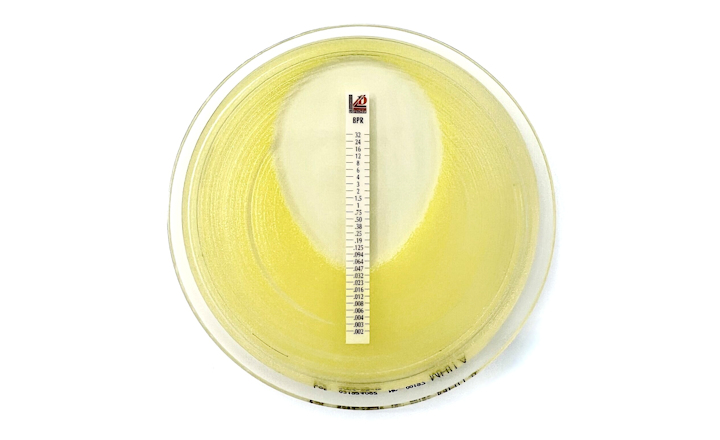
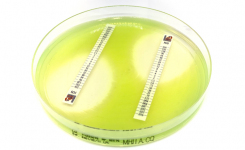
Liofilchem has received clearance from the FDA to market the Ceftobiprole 0.002-32μg/mL MTS™ (MIC Test Strip) in the United States, a patented quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of Ceftobiprole.
MTS™ BPR can be used to determine the MIC of ceftobiprole against the following microorganisms for which ceftobiprole is active clinically and/or in vitro according to the FDA drug approved label:
- Escherichia coli
- Klebsiella pneumoniae
- Staphylococcus aureus (includes methicillin-resistant isolates)
Ref. 921401 - 10/pack (individually packed)
Ref. 92140 - 30/pack (individually packed)
Ref. 921400 - 100/pack (canister pack)
The MTS™ range is also compliant with MDSAP (Australia TGA, Brazil ANVISA, Health Canada, USA FDA, Japan MHLW/PMDA) and is registered as a clinical diagnostic device at the competent Authority in many countries outside Europe.
Click here for further information on US FDA-cleared MTS™ items or use the Request Information button below.