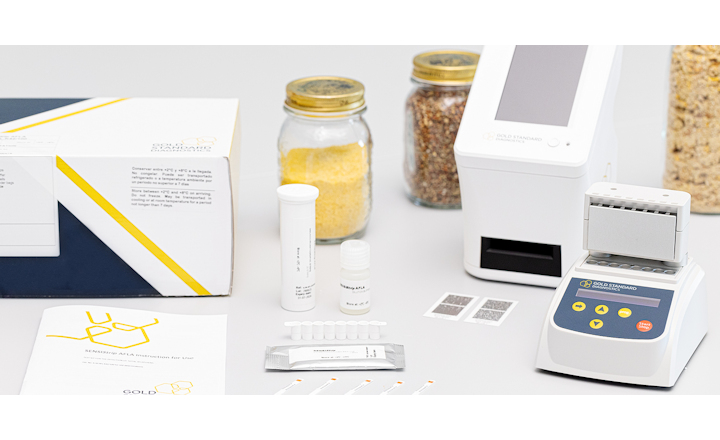
Charm Sciences' lateral flow MRLAFMQ test for the detection of aflatoxin M1 in raw commingled milk is the first lateral flow test to receive independent third party validation. The peer reviewed report of the validation by Technology and Food Science Unit of the Institute for Agricultural and Fisheries Research (ILVO-T&V) has been published by the Taylor & Francis Group.
Aflatoxin B1, the most toxic aflatoxin and a known carcinogen, can be passed from contaminated feed through a cow’s milk as aflatoxin M1, to a consumer. While aflatoxin M1 is not as potent as B1, it is still classified as a group 1 carcinogen.
"Unfortunately, there is no known way to extract aflatoxin M1 from milk," said Bob Salter, Vice President of Regulatory Affairs for Charm Sciences, "so it is vital to have an effective screening plan in place. Not only does Charm’s MRLAFMQ lateral flow test detect aflatoxin M1 in only 15 minutes, but it requires no preparation of materials, making it extremely easy for plants to run consistently. This test is easily run on the same Charm® equipment used to screen milk for antibiotics at most dairies and farms."
The test kit was evaluated with both Charm’s ROSA® Reader and the Charm EZ® system. The all-in-one Charm EZ system identifies the appropriate mycotoxin test using color-coded strips then reads and displays the quantitative test results; test data is stored on an SD card for reporting and analysis.
The article Validation of a lateral flow test (MRLAFMQ) for the detection of aflatoxin M1 at 50 ng/L in raw commingled milk is available for download from Taylor & Francis online at
http://www.tandfonline.com/doi/pdf/10.1080/19440049.2014.979888