- Manual colony counting is highly prone to human error.
- Errors from miscounts, as wells as manual data entry are exacerbated when humans are faced with large volumes of samples and time pressure.
- APAS Independence offers AI-enabled culture interpretation and digital tools to improve current plate counting practices.
- Direct integration with LIMS removes transcription errors and enhances data integrity.
It’s no secret that microbiology can be an imprecise art. Numerous studies1,2,3 have already highlighted the error-prone nature of manual colony counting - particularly the variation between readers which can be up to 50% in positive plates but is also surprisingly high even in true negative plates!
Colony size, colour, growth patterns, location on the plate, as well as ambient lighting conditions all affect accuracy in plate counts. Miscounting is not the only concern, however - another potential source of error in manual methodology is the accurate recording of results, with transcription errors further contributing to uncertainty in data records. This creates a challenge for pharmaceutical manufacturers, with no long-term storage of culture plates available for review of environmental monitoring results that contribute to batch release records.
Clever Culture Systems’ APAS Independence® is an innovative platform technology that automates culture-plate screening and interpretation. The system uses artificial intelligence to provide a robust solution for automated plate enumeration, successfully detecting growth 100% of the time with a low false positivity rate of 7.3%4, and producing counts in high agreement with human reads, with correlation coefficients of >0.97 for counts of 0-50 CFU5.
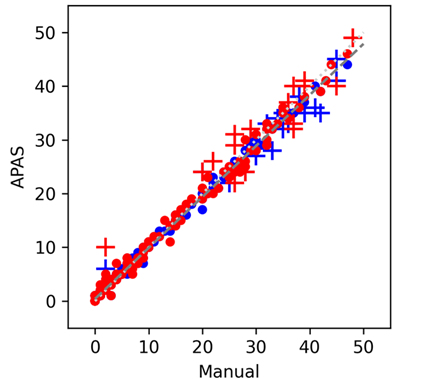
Comparison of APAS counts versus manual counts
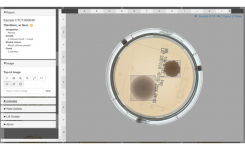
As with manual counting, spreading organisms and crowded colonies do present a challenge. Where human review is required the APAS Web User Interface assists users with its counting tool, providing both top and bottom-lit lighting modes to maximise colony detection and differentiation. In a study assessing the performance of APAS Independence, the variation between microbiologist reads using this counting tool was less than 10% for counts of 0-250 CFU5.
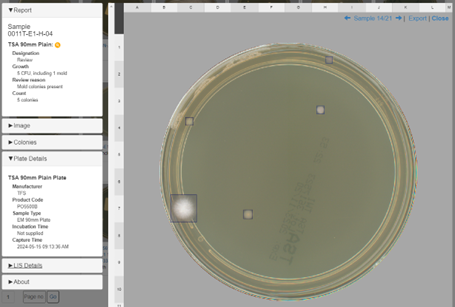
APAS Independence automated colony detection and reporting
With its seamless integration and reporting of results to LIMS, APAS Independence minimises the risk of transcription errors and provides audit trail logging for full compliance with Annex 1 and CFR 21 Part 11 data integrity and traceability requirements.
To learn more visit Clever Culture Systems or use the Request Information button below to connect directly with the supplier.
References:
- Accuracy of Plate Counts, Scott Sutton, Journal of Validation Technology, 2011
- Ready for the count? Back-to-basics review of microbial colony counting, Tim Sandle, 2020
- Detection of Small Events in Environmental Monitoring Culture Media, Laurent Leblanc, Katia Imhoff, BioMérieux, 2019
- Pilot secondary validation study data, July 2023
- CCS Linearity and accuracy study data, April 2024