- Order by AstraZeneca to purchase five APAS Independence instruments for environmental monitoring plate reading
- Installation of first five instruments expected within the next six months
- Commercial traction is building with multinational pharmaceutical customers
Brent Barnes, CEO & Managing Director said:
“The purchasing agreement demonstrates the value the APAS technology provides and unlocks an initial roll-out of five instruments across a number of their large manufacturing operations. This decision was made based on demonstrated performance of the technology within the AstraZeneca manufacturing processes and provides credibility for the technology more broadly.
This milestone provides evidence and confidence that the APAS Independence is a fully validated technology that meets the stringent requirements for environmental monitoring during drug manufacturing, applicable to all customers globally for this application. Pleasingly, evaluations with additional multinational pharmaceutical customers are expected to commence in the current quarter.”
AstraZeneca agreement to purchase five APAS® Independence Instruments.
The Company has signed an agreement with AstraZeneca for the purchase of five APAS Independence instruments, including the provision of annual support and maintenance services for the next seven years. The five instruments will be progressively installed, scheduled to occur over the next six months.
In January 2023, the Company partnered with AstraZeneca to develop a new APAS analysis module for the reading of culture plates used in environmental monitoring1. In March 2024, Clever Culture Systems launched the APAS Independence in the pharmaceutical market after completion of its formal validation of the system demonstrating the performance of the system2.
Summary of results:
The company’s primary validation program was designed to meet the pharmacopeial requirements for the validation of alternative microbiology methods used in pharmaceutical manufacturing. These requirements define various tests that assess the performance of the technology to the current compendial method. In this case, manual reading of the culture plates by two microbiologists is the existing method. The data generated in the study establishes a key body of evidence required by customers when seeking to adopt the technology.
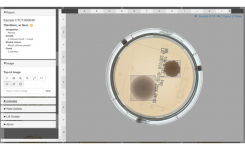
- Over 35,000 plate images captured, 40,000 microbiologist plate reads and approximately 3 million colonies counted
- 9 microbial organisms tested across a broad range of operational conditions and practices
- All performance targets for the technology successfully achieved:
Primary performance target: 0% Qualitative False Negative Rate - No plates with microbial growth missed by APAS® PharmaQC during testing
Secondary performance target: High counting accuracy and linearity demonstrated for standard organisms
Commercial traction building with multinational pharmaceutical customers
The Company’s strategy has been to target large multinational pharmaceutical companies, who have the potential to deploy multiple instruments across their manufacturing network. Through this targeted approach, the Company has created a strong pipeline of opportunities within this customer segment, and recently completed installations with two multinational pharmaceutical customers for evaluation of the technology within their manufacturing process.
Use the Request Information button below to contact Clever Culture Systems.
1. https://www.lbtinnovations.com/wp-content/uploads/9-January-2023-AstraZeneca-Partners-LBT-for-APAS%C2%AE-Pharma-Development.pdf
2. https://www.lbtinnovations.com/wp-content/uploads/APAS-PharmaQC-Commercially-Ready-Performance-Finalised.pdf