Environmental monitoring plays a vital role to support the manufacturing of safe medicinal and other regulated products. Traditional methods include the use of 90mm settle agar plates and smaller 55mm contact (or RODAC) plates, which remain the gold standard for the viable sampling of air, surfaces and personnel. Results from this sampling can provide essential information about the state of contamination of the environment throughout various stages of production.
New microbiology tools are emerging that automate the reading and reporting of agar plates collected from environmental monitoring. These new methods typically capture a plate image and use advanced digital tools to interpret growth on the plate and identify individual colonies. This helps improve overall manufacturing processes by offering:
- Greater traceability and data integrity of every result
- Improved throughput and efficiencies for releasing environmental monitoring results
- Fully digital workflows enabling a paperless environment
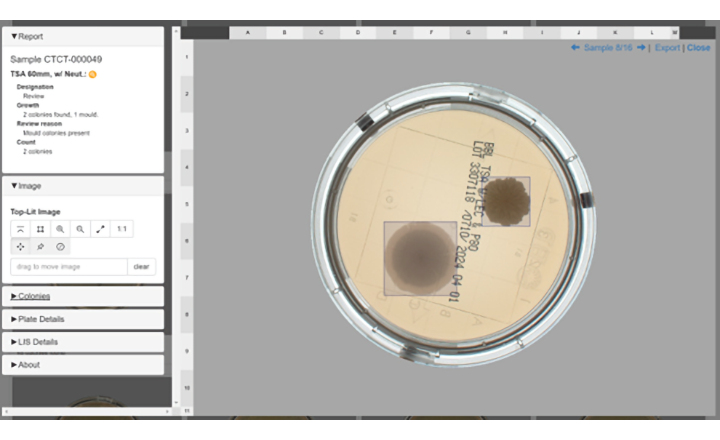
Clever Culture Systems (CCS), APAS Independence technology is an open platform with a flexible design able to process and read any plate type commonly used in environmental monitoring. The technology provides highly accurate colony differentiation and counting, including automated flagging of any moulds or spreading organisms present on the plate.
Building on an established validated platform, the APAS technology is now available to automate reading of contact plates, in addition to the existing 90mm plates. Importantly, this is supported on a single hardware platform. With the addition of the contact plates, APAS Independence is now the only fully automated technology capable of providing high-throughput bulk processing of both contact plates and 90 mm plates used for environmental monitoring in pharmaceutical settings.
The APAS Independence’s open platform is a flexible solution designed to be easily integrated with your existing microbiology workflows. With the added contact plate application, APAS Independence offers a single automation solution for your microbiology laboratory. Key benefits include:
- Bulk plate loading / unloading and processing without the need for adaptors
- High-throughput automation at 200 plates per hour
- No proprietary media, validated on plates from major media manufacturers
- Complete digital microbiology solution with image retention for review
- Extensive performance validation completed following compendial guidelines
- Compliant software with full audit trail and digital approvals
As regulatory pressures increase, intelligent and flexible automated plate reading solutions are an important consideration for your laboratory. APAS Independence integrates into your current processes, allowing you to maintain your existing validated protocols. You can continue using your established culture media and incubation equipment while APAS Independence handles the automation of plate analysis and result documentation.
Interested in learning more? Get in touch using the Request Information button below.