For pharmaceutical companies, maintaining strict environmental monitoring compliance is not only a regulatory checkbox but also a vital protection for product safety and brand reputation in the high-stakes field of pharmaceutical manufacturing.
Artificial intelligence (AI) is now enabling automated collection and data interpretation of environmental monitoring samples, producing accurate, usable data for quick decision-making, and improving data integrity and compliance using auditable electronic records.
Clever Culture Systems (CCS) has recently introduced the APAS® Independence (Automated Plate Assessment System), specifically designed for automating and interpreting the reading of environmental monitoring (EM) culture plates, which are widely used in sterile manufacturing environments. In this conversation with Dr Steven Giglio, Scientific Director of CCS, we explore the junction of EM compliance with novel AI-driven technology. We will cover how this innovative technology transforms the fundamentals of compliance in pharmaceutical manufacturing.
Q: Environmental monitoring of cGMP facilities is a big part of regulatory compliance. What are the challenges that most facilities face?
Steven: Requirements for EM are stringent, especially in Grade A environments. There has been increasing scrutiny on counting and reporting EM culture plates with several 483s highlighting issues including procedures used to read plates, qualification of staff, and adequate record keeping (including traceability) of critical results. While most of the work for counting EM plates is performed in a microbiology laboratory, the microbiologists themselves are often tasked with additional activities, and so these skilled staff are being utilised more than ever.
When you consider that most microbiologists working on EM plates in a sterile production environment are looking at no growth (zero CFU, typically greater than 95% of culture plates) and best practice requires 2 independent reads of each plate, there is a significant amount of skilled time spent reading plates with no microbial growth present. With increasing daily pressures, it is inevitable that human errors will occur, in counting and transcription, compromising data integrity and potentially product safety.
Q: In what way can Clever Culture Systems APAS Independence be a solution?
Steven: The APAS Independence automates imaging, analysis, and interpretation of microbiology culture plates as part of routine EM procedures. The system will provide segregation of culture plates with growth and no growth, in addition to providing an estimate of CFU and flagging plates with mold present. The plates with no growth do not require any further interaction with the microbiologist, and those plates that are non-zero CFU (typically <5%) can be actioned in accordance with local laboratory procedures. The culture plate results are electronically generated and can be directly integrated with laboratory information management systems (LIMS) allowing compliance with 21 CFR Part 11. The throughput of the system is approximately 200 plates per hour.
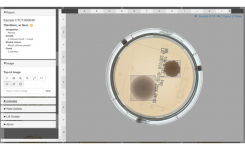
So, the solution is several fold. Firstly, a large amount of time reading EM culture plates is removed. No growth results are automatically reported to the LIMS and microbiologists can focus on the truly skilled art of microbiology involving actual plates with growth! This saved time could be utilised in additional training, investigations, or other value-add tasks. Secondly, automated systems integrated with the LIMS eliminate any transcription errors, significantly derisking non-compliance and strengthening data integrity across the batch release chain. Thirdly, the system has a full audit trial and log which allows investigation of instrument interactions by operator, thereby facilitating any investigational activities. Also supporting this activity are the plate images available by the APAS system – the images are high quality and representative of plate-in-hand. This allows a digital review of plates to support colony counting and morphology assessment, including a number of image manipulation features to allow detailed interrogation. Here is an example of APAS Independence Colony Detection and Reporting
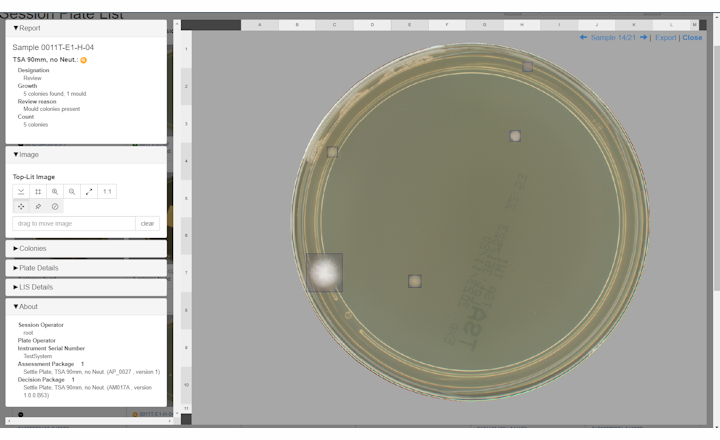
Q: How does the APAS Independence assist in identifying anomalies in environmental data that humans might miss?
Steven: APAS Independence is a validated technology that has been stress tested according to compendial guidelines (USP <1223>, Ph Eur 5.1.6) with primary validation data supporting a zero false negative rate for detecting plates with growth at five days of incubation. There is little data that discusses the false negative rate of manual reading by humans, however initial studies with the APAS Independence have observed occasions where the system has identified growth previously missed by operators in a routine setting. Therefore, we are confident the system will be non-inferior to the current manual reads and will, therefore, help facilities improve the consistency and reliability of counting EM plates without compromising this important data point.
Further, the introduction of automation acts as a catalyst of change to revisit any internal systems and processes that could provide further opportunities to reduce data integrity risks through process optimisation. A classic example being microbiologists writing on plates. Implementing APAS Independence, with the use of the one of many EM software systems, provides a full end-to-end automation solution where the manufacturer side barcode can be linked to trace EM data to specific sampling sites and therefore remove the need to write on plates which is a transcription risk also. The automated plate reading process can therefore close the loop and serve to reduce result data discrepancies and ensure accuracy of the final reported result.
Q: What are the key regulatory hurdles facing the implementation of the APAS Independence for environmental monitoring?
Steven: At CCS we have been the pioneers in AI and microbiological culture plate reading with over 10 years of experience, with FDA and CE cleared software in the diagnostic sector. This background and the strength of the primary validation data significantly de-risks the APAS application and has certainly given confidence for users to uptake the technology. Importantly, introduction of the APAS Independence integrates with the existing ways of working and does not require any broad microbiological or process changes – there is no need to validate any changes in incubation time or temperature, or proprietary media. The microbiological performance validation task is therefore much simpler, with only a head-to-head comparison of the APAS v human read result needed to demonstrate non-inferiority, notwithstanding that some sites may wish to repeat some compendial tests or challenge the system in a unique way.
AI is also a buzz word that instantly raises some concerns with regulators. Our history of working with global regulators, means we have a large amount of heavily scrutinized documentation and data that describes the use of the technology and ensures the instrument is safe and performs to the high-level users expect. Our development processes are highly structured and controlled in line with Good Machine Learning principles and AI governance models, consistent with the ISPE AI maturity model.
So, whilst the microbiological validation is a critical piece of the implementation puzzle, a number of considerations involving multiple stakeholders (microbiology, quality, production, IT) is clearly required. CCS have experts across all these disciplines to facilitate any integration process.
Q: Can you share a user experience where the APAS Independence significantly improved environmental monitoring outcomes for a company?
Steven: EM culture plate reading has been high on the agenda for many pharmaceutical companies for a long time. While there are several other systems available, AstraZeneca approached us to explore a collaborative effort to deliver a solution suitable for industry. Their challenges were not unique, in that a considerable proportion of the EM culture plates used in sterile production sites are negative, >98% of the time, and that each plate is read by 2 microbiologists. AstraZeneca were committed to delivering a platform solution that could be deployed across their various manufacturing sites. This was managed through their Global Modernisation group, who developed user requirements and have worked closely with us throughout the development process. We then worked to those requirements, as a minimum, to deliver what we have available today.
One of the key considerations was scalability in that they required a single technology that could assist smaller sites whilst also accommodating large sites processing >1,000 plate per day. Another key requirement was that the introduction of automation should not significantly disrupt the existing ways of working across a global network of laboratories. As already mentioned, APAS Independence really does assimilate into the role of a microbiologist plate reader without any change in existing workflow, and its capacity to read 200 plates per hour delivers a scalable solution with high-throughput.
Q: Can the APAS Independence improve sustainability and bottom-line profitability?
Steven: There are two key areas where APAS Independence assists companies to improve efficiency of operations and de-risk processes helping to improve overall profitability and sustainability of operations.
Firstly, we know that staffing challenges exist for many companies and microbiologists are often pushed for deliverables on strict timelines and in some cases resourcing EM plate reading is a challenge. Introduction of APAS Independence helps improve laboratory efficiency, ensure EM plate reading is completed on time, and most importantly frees up bandwidth for other activities. This additional resource availability is likely to translate to additional cross-functional training, performing GEMBA walks in classified areas, or finishing investigations on time.
Secondly, the fact that a fully integrated automation system which has an end-to-end auditable result trail also underpins the data integrity required for EM programmes. A number of 483s unfortunately point to issue with the quality of data and record keeping for EM culture plates. The 483s have a reputational cost which cannot be quantified directly or accurately, but there is also an operational cost that will involve the entire organisation, at a significant cost in terms of time and money. In this regard, the APAS Independence is likely to provide a robust solution that reduces the risk of future regulatory observations, supporting ongoing compliance.
Q: Why APAS Independence? How does it compare to other similar systems on the market?
Steven: As an end-point reader, the APAS Independence does stand alone as a fully validated automation solution capable of reading both 55mm and 90mm EM culture plates. As a result, the integration and validation effort with the APAS Independence is unlikely to be as large as some other systems as the system is not impacting on any other existing processes such as time or temperature of incubation. Further, there are no proprietary media used, which means users have the flexibility to choose and change between the major manufacturers. This is especially important during supply issues as the system can be validated to work with a Company’s primary and secondary culture media supplier.
The APAS Independence does not need or have an incubator either, meaning there really are no restrictions on the volume of plates that could be processed in a day; the only real restriction is the time to process plates, which is 200 plates per hour, meaning one system can readily process over 2,000 plates in a single day. I do not think this is a restriction at all really, it is at least three times faster than a microbiologist and supports the processing needs of many facilities. Of course, if you do need more processing power you can always have multiple APAS Independence to increase capacity.
One of the most common feedback on our system is the quality of the images and that they are truly representative of a plate-in-hand view. All our IP is in our imaging system to produce images that are free from interferences and reflections, which assist with our proprietary AI application. Images are available through the systems interactive Web-UI tool which can be accessed from any computer within the same network as the instrument. There are several image manipulation tools available which allow users to toggle between top- and bottom-lit images, count colonies, zoom in and out, and apply AI-bounding boxes to highlight detected colonies. All these features provide an interactive digital microbiology experience to the user, offering an elevated level of image engagement functionality which customers rate very highly.
Q: How do you envision the future of AI in pharmaceutical compliance? What considerations do company’s need to be wary of when seeking to apply AI based technology?
Steven: I really do think this space has a watching brief as AI matures and as new AI modalities emerge. In the medical device sector, there are several guidelines to establish the framework for good AI governance, which is seeing growing adoption and several cleared products on the market. Users are looking for more direction on the details of execution. This is not something that is expected from an industry or regulatory group, but it is always the execution and data management (and data cleanliness) that causes the most problems, despite the relatively simple conceptual framework.
Generating an AI model takes a specific skillset to develop and validate, its not a trivial task. The purpose of the model needs to be explicitly clear, and it cannot be expected to do too many things. The more tasks you require a model to do, the greater the complexity, and the greater the burden of proof required across multiple functions. There is inevitably a compromise in performance or function if the user requirements are too broad.
There are already some notable examples of AI in the manufacturing process being used for documentation review using language learning models – a great way to review batch records for completeness and inconsistencies. Some vision systems provide real-time AI monitoring of fill lines, looking for correct volumes, capsule integrity, or other known manufacturing non-compliances.
AI is good at doing repetitive tasks with predictable outcomes, in a consistent way, and it is these types of application where AI is best suited. This means you will always, yes always, need people to do jobs and their knowledge and expertise is still critical to the organisation. However, the introduction of any AI technologies really does allow organisations to mature and grow with existing workforces and should be embraced but carefully planned and communicated at all levels.
Improve Your Environmental Monitoring Compliance With AI: Schedule a Demo of the APAS Automated Plate Assessment System. Use the Request Information Button below.
About Dr Steven Giglio: