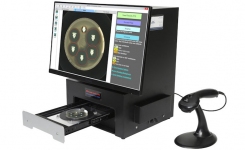
The BIOMIC V3 system automates the reading of antibiotic disk diffusion following CLSI/EUCAST/BSAC guidelines it can be used with Etest (bioMerieux), MIC Test Strips (Liofilchem) and 96-well microtiter plates.
The following 2016 guidelines are now updated in the BIOMIC V3 software:
CLSI - Clinical Laboratory Standards Institute
- M100-S26: Performance Standards for Antimicrobial Susceptibility Testing
- M45-Ed3: Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria
- VET01S-Ed3: Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals
EUCAST - European Committee on Antimicrobial Susceptibility Testing
- Clinical Breakpoint Tables for Interpretation of MICs and Zone Diameters v 6.0
- Quality Control for MIC Determination and Disk Diffusion v 6.1
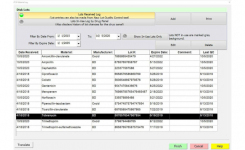
BIOMIC V3 can use a bar code entry and a 2-Way LIS/LIMS interface optimizing lab workflow. The system can also automate microbial identification from API® (bioMerieux), RapID™ (Remel), BBL™ Crystal™ (BD), Liofilchem® ID Systems, colony count and chromogenic agar plates.
Please note : Any products described on this page are
for Research Use Only and not intended for clinical diagnostic procedures unless otherwise stated.