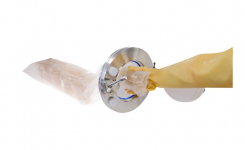
As part of its continued commitment to customer satisfaction and ISO9001:2008 Quality Management, Cherwell Laboratories conducts regular surveys to identify potential areas for improvement and also following significant product changes. In the most recent survey, completed by customers using Redipor® Prepared Media products packaged in Cherwell's new laminated flow wrap, overall customer satisfaction levels were high. The new bi-layer laminated film wrap, designed for automated packaging, minimises product handling and enhances product security, as well as reducing the potential for failures from manual heat-sealing. The high clarity packaging also ensures better visibility of plate condition and labelling through intact packaging, thereby maintaining sterility and shelf life.
Excellence in customer service and products is at the core of Cherwell's company values, and the company always aims to work with customers to ensure they receive the best products to meet their specific needs. It is for this reason that Cherwell undertakes a customer survey every time there is a significant product change. Whilst the majority of customers surveyed were satisfied or very satisfied with the new flow-wrap packaging, Cherwell has been able to work with individual customers to address any concerns and, where applicable, provide an alternative solution.
"Whilst we are proud to achieve high satisfaction levels, we will continue to further improve our products and service levels to ensure the on-going satisfaction of all our customers" said Andrew Barrow, Sales Manager, Cherwell Laboratories. "Furthermore, since we offer bespoke solutions, if any of our standard products are not suitable, we will work with our customers to meet their exact requirements."
The Redipor range of prepared microbiological media products includes Petri dishes, contact plates and gamma irradiated media, as well as bottled broths, diluents and infusion bags. All products are manufactured under the control of an ISO9001:2008 registered Quality Management system and are available in flexible quantities, packaging options and even formulations.
For more information about Cherwell Laboratories, please visit www.cherwell-labs.co.uk or follow @CherwellLabs.