Diagnostic assay development is a critical process in the field of healthcare and biotechnology, aimed at creating tests that can accurately detect and diagnose various diseases and conditions. This development involves the use of specific materials, reagents, and the expertise of Contract Development and Manufacturing Organizations (CDMOs).
In Vitro Diagnostic (IVD) assay development and commercialization present challenges, but companies such as Zeptometrix® offer custom controls and raw materials to support the process of creating fit for purpose diagnostic assays.
In this special focus, Andrew Zenger, Global Product Manager at ZeptoMetrix, explains how third-party molecular controls are key to establishing clinical laboratory quality practices and ensuring regulatory compliance. Andrew also outlines considerations to bear in mind when selecting a supplier of third-party QC materials and explains why assay developers choose to partner with ZeptoMetrix throughout the assay development process.
Diagnostic assay developers can use inactivated, whole-organism QCs for antigen tests to better evaluate their assays' performance. A great example is the ZeptoMetrix PROtrol™ product line, designed for antigen-based diagnostic methods for infectious diseases, including lateral flow immunoassays.
Lyophilising your reagents as robust beads with a precise and constant dose offers significant advantages. These include enhanced test accuracy, production efficiency, and ease of transportation and storage. Discover the advantages of Biofortuna's lyophilised beads in this fascinating video, and download the application note for more details.
Assay developers will be interested in reading PCR Biosystems' latest product bulletin, which is now available for download. It outlines the key features and benefits of air drying and the performance of Air-Dryable Probe 1-Step Mix in both singleplex and multiplex assays before and after drying.
Building on its vast experience in manufacturing advanced therapies, Cytiva is tackling the industry challenges that hinder patient access and wider adoption of autologous CAR T cell therapies with the new Sefia cell therapy manufacturing platform.
Read about Gold Standard Diagnostics range of magnetic separation beads and latex agglutination tests to purify and detect E. coli O157, other STECs (Shiga Toxin-producing E. coli), and Shiga Toxins.
Maximize sample collection and elution with Puritan® Hydraflock® swabs. Specifically engineered for a wide range of biological specimens. HydraFlock’s three-dimensional microstructure design facilitates greater specimen collection. The multi-length fibers have a greater absorption area than traditional perpendicular nylon flock fibers for more rapid elution and maximal sample preservation. Ideal for a variety of clinical settings and diagnostic tests.
Third-party Molecular Controls Are Crucial to Accurate Testing in Clinical Laboratories
In this interview with Andrew Zenger, the Global Product Manager at ZeptoMetrix, we learn about how third-party molecular controls are key to developing clinical lab quality practices, regulatory compliance, and factors that labs should consider when choosing a supplier of third-party QC materials.
Find Out More
How Do You Evaluate and Monitor the Performance of Antigen-based Assays?
ZeptoMetrix® PROtrol™ is a new line of products designed for antigen-based diagnostic methods for infectious diseases, including lateral flow immunoassays.
Find Out More
Custom Lyo Beads from Biofortuna - Precision at Scale
Lyo beads are expanding possibilities in point of care assay development. As well as obviating the need for cold chain they improve assay accuracy and lower the cost per test. See how the Biofortuna contract manufacturing service delivers unrivalled scale, precision and accuracy with this case study.
Find Out More
Air-Dried Assay Development: A Simple, Fast and Cost-effective Option to Dry Molecular Assays In-house
Assay developers are increasingly moving towards dried formats to alleviate the costly cold-chain shipping and storage demands of traditional 'wet' qPCR-based diagnostic tests. Air-drying has emerged as a simple, fast and cost-effective option for drying down molecular assays.
Find Out More
A Next Generation Cell Therapy Manufacturing Platform from Cytiva
Sefia Select™ system comprises two hardware components, specialized application software, and single-use kits to provide a closed and automated solution to your cell therapy manufacturing needs; resulting in an efficient, secured and standardized process.
Find Out More
Microgen Latex Agglutination Kits for Salmonella, Legionella or Staphylococcus aureus ID
Get fast and reliable results confirmed in 2 minutes for confirmatory identification of Salmonella, Legionella or Staphylococcus aureus colonies in food and environmental samples.
Find Out More
Collect a Better Sample with Puritan® HydraFlock® Flocked Swabs
Why use flock? Flocked swabs collect more and also elute the maximum available specimen into transport media, as compared to spun fiber swabs and patented HydraFlock® takes that a step further with its very unique fiber structure.
Find Out More
Cambrex Nears Completion of 5-year, $100-Million-Dollar Investment Plan
Cambrex is nearing the completion of a 5-year, $100-million-dollar investment strategy, one year ahead of schedule, adding more than 150,000 square-feet of capacity and capability expansions across 70% of its North American and European drug development and manufacturing network.
Find Out More
Lateral-Flow Assay Development: Its Not Rocket Science But You Need Help - rapidmicrobiology Podcast
In this podcast, Andre Alfaro, Director of Assay Development at nanoComposix, lends expertise to medical device manufacturers entering the LFT market, particularly for COVID-19. Andre provides insights into this fast-growing sector and explains how nanoComposix can help every step of the way.
Find Out More
BBI and BioAgilytix Partner to Streamline and Simplify Bioanalytical Testing
Drug developers often need a variety of antibody reagents tailored to their candidate therapy and for a range of testing applications. However, developing and securing a reliable supply of fit-for-purpose, customized antibody reagents is not easy. The new partnership will address this challenge.FDA Clearance MDx-Chex® for BC-GP and BC-GN Molecular Diagnostics QC for Bloodstream Infections
Provided in a comprehensive and easy-to-use format, MDx-Chex evaluates the entire analytical process of the Luminex VERIGENE® BC-GP and BC-GN Panels for sepsis, including cell lysis and DNA extraction, as well as DNA hybridization, detection, and analysis.Novel Engineered Polymerases for the Detection of Infectious Diseases
This webinar is perfect for researchers, healthcare practitioners, lab staff, and anyone interested in the latest medical diagnostics developments to better understand engineered polymerases' fundamentals and their vital role in infectious disease diagnostics.Affordable Storage Tube Sealing Device
The Semi-Automated Septum Cap Capper from Azenta Life Sciences is a storage tube sealing device designed to preserve sample integrity and audit trails in biobanks, compound libraries and other high-throughput storage applications.
Find Out More
Micro Flow Meter and Degasser Set-up for Nanoparticulate Formulation Dispensing
Biotech Fluidics reports on the successful development and implementation of a pioneering combined micro flowmeter and degasser set-up to improve dispensing of a nanoparticulate formulation for a leading medical technology company.
Find Out More
Enzyme Substrates Toolbox. A Signalogenic Guide Part 1 - Chromogenic Substrates
Free downloadable ebook explains the science behnd chromogenic substrates, how to select the optimal substrate for your research, and gain practical tips for using enzyme substrates with initial experiment details.
Find Out More
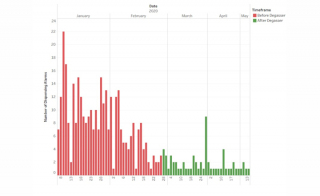
Degassing System Reduces Anomalies in Diagnostic Kit Reagent Dispensing
Biotech Fluidics report how installation of two DEGASi® Plus semi-prep 6-channel degassing systems on a production line, has enabled a diagnostic kit manufacturer to almost eliminate inaccurate reagent dispensing issues.
Find Out More
Fapon Delivers Customized IVD Solutions for the US Market at ADLM 2024
At ADLM 2024, Fapon showcased its innovative technology platforms, including antibody discovery, protein expression, cell fermentation, and protein purification and analysis.
Find Out More
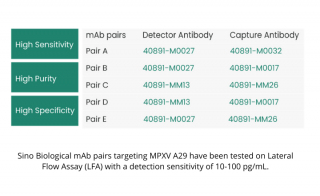
Accelerate Mpox Research With Sino Biological MPXV Proteins and Antibodies
Sino Biological is at the forefront of accelerating Mpox research, offering a comprehensive portfolio of MPXV proteins and antibodies: various recombinant MPXV proteins with different tags, over 30 antibodies targeting various MPXV antigens, validated for applications such as LFA, ELISA, and WB assays.
Find Out More