The BIOMIC V3 Microbiology System and MALDI-TOF Mass Spec Systems, including the Maldi Biotyper (Bruker) and VITEK MS (bioMerieux), provide a complete ID-AST solution for the microbiology lab.
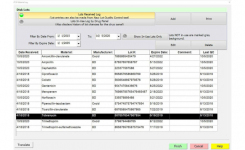
MALDI-Link™ middleware is an optional feature of BIOMIC V3, directly interfacing Maldi Biotyper (Bruker) and BIOMIC V3. BIOMIC V3 automatically accepts output from Maldi Biotyper, saves it to the BIOMIC V3 database, and transfers the final ID and specimen identifier to the LIS/LIMS. MALDI-Link™ middleware eliminates manual transcription errors and an interface between Maldi Biotyper and LIS/LIMS.
BIOMIC V3
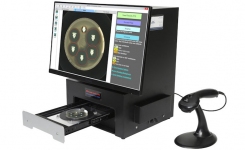
BIOMIC V3 is an open system digitally automating CLSI and EUCAST clinical microbiology tests and QC from various manufacturers. BIOMIC V3 systems are customized with optional modules: Disk Diffusion, 96-Well Microtiter, MIC Strip, Organism ID, Colony Count, Agar Dilution, and Urine Screen.
BIOMIC V3 provides a digital record of test results and high-resolution images. An LIS/LIMS interface combined with bar code reading and touch-screen entry on a 24-inch monitor offers labs an optimal setup to standardize, record and report test results. BIOMIC V3 is designed and manufactured by Giles Scientific in Santa Barbara, California, USA.