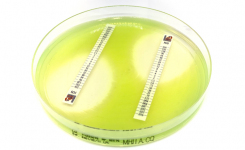
MTS-SAS combines the reliability of MIC Test Strip with patented tools to facilitate and standardize a critical assay such as the in-vitro combination of antibiotics.
Antimicrobial resistance is a major challenge for clinicians and clinical microbiologists, the use of synergy testing is today increasingly requested.

MTS-SAS procedure is significantly faster than any other methods in antibiotic synergy tests (i.e. time-kill, checkerboard), does not require any additional trainings to those who are skilled in the usage of MIC Test Strip, reduces or eliminates the risks of procedure errors when compared to laborious and complicated traditional methods.
Find out more:
- Product literature
- Product technical sheet
- US patent US9365886B2 A device for standardising the in-vitro synergy testing of two antibiotics through the method of crossing the gradient strips. (Liofilchem, 2012).
Planning to visit ECCMID
- come and meet us at stand # 1.97A