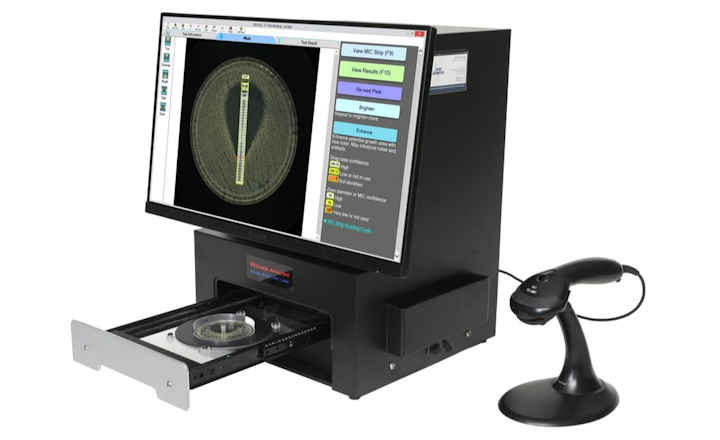
Q-linea AB have announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance for the company's ASTar® System, enabling market launch to hospitals and laboratories in the United States.
Stuart Gander, CEO of Q-linea, said: “The approval is a significant step for Q-linea in the infectious disease diagnostics field in the U.S. Feedback from hospitals that have had early access to ASTar has been very positive, and we are excited to now be able to bring the system to labs across the U.S., which will make a real difference for patients with severe blood stream infections. We are pleased with the panel of drug-bug combinations which has been approved.”
According to the Centers for Disease Control and Prevention, at least 1.7 million adults in the United States develop sepsis each year and nearly 270,000 die as a result. ASTar enables rapid therapeutic response to sepsis directly from a positive blood culture in approximately six hours, giving physicians the tool needed to improve patient outcomes and reduce mortality.
Features and Benefits of the ASTar® System Include:- Full Automation - analyzes up to 12 samples in parallel with random-access loading and less than two minutes hands-on time
- True MIC results - ~ 6 hours true MIC results are assured through controlled inoculum, and 7-11 consecutive doubling dilutions of each antimicrobial in panel
- Comprehensive AST panel - With over 330 wells available for antimicrobials, the AST disc is designed to facilitate future panel expansion.
For more information visit qlinea.com/us