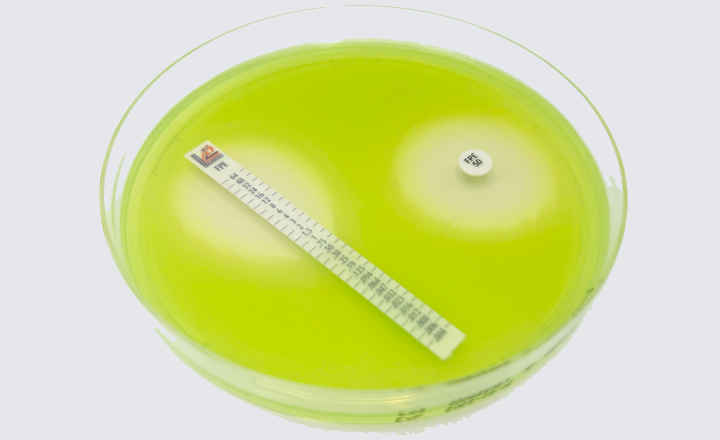
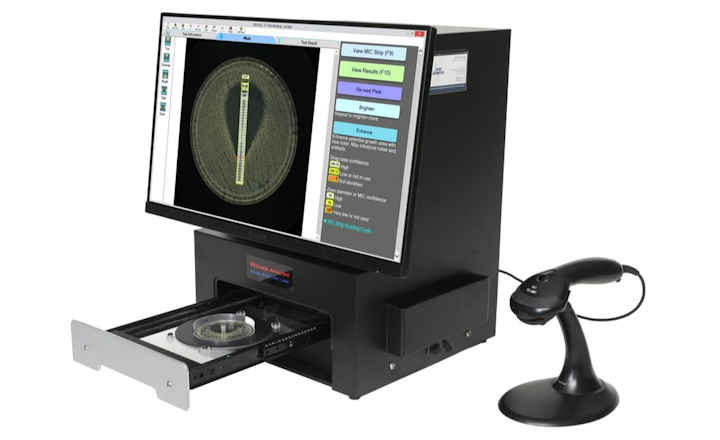
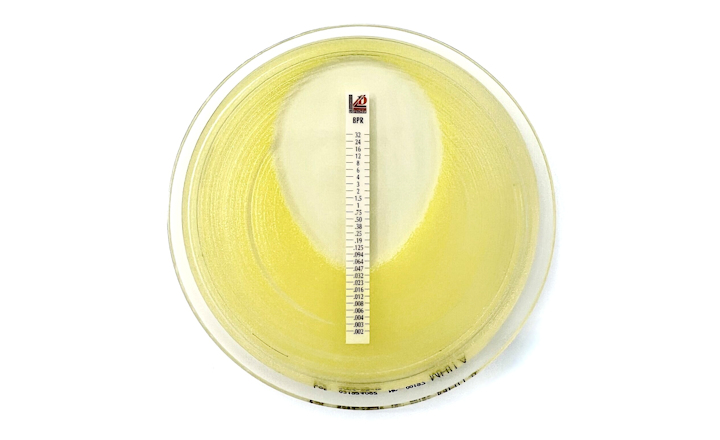
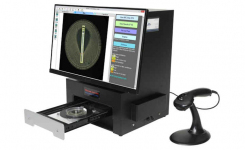
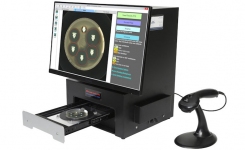
BIOMIC® V3 is a digital imaging system for automating the reading and interpretation of AST and ID tests from various manufacturers.
The following guidelines are now updated in the 2020 BIOMIC® V3 software
- CLSI (Clinical Laboratory Standards Institute) Guidelines: M100, 30th edition: Performance Standards for Antimicrobial Susceptibility Testing
- EUCAST (European Committee on Antimicrobial Susceptibility Testing) Guidelines:
- Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0
- Expert Rules - Intrinsic Resistance and Unusual Phenotypes, Version 3.2
- Zone diameter breakpoints for rapid antimicrobial susceptibility testing (RAST) directly from blood culture bottles, Version 1.1
Visit www.biomic.com to learn more.