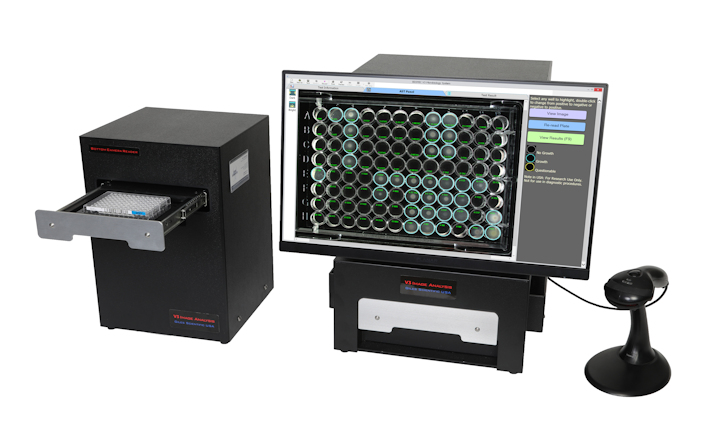
BIOMIC V3 is an open system utilizing digital imaging to automate the reading and CLSI and EUCAST interpretation of clinical microbiology tests and QC from various manufacturers.
Systems are customized with optional modules including disk diffusion, 96-well microtiter, MIC strip, organism ID, and colony counting.
BIOMIC V3 provides a digital record of test results and high-resolution images. An LIS/LIMS interface combined with bar code reading and touch-screen entry on a 24-inch monitor offers labs an optimal setup to standardize, record and report test results.
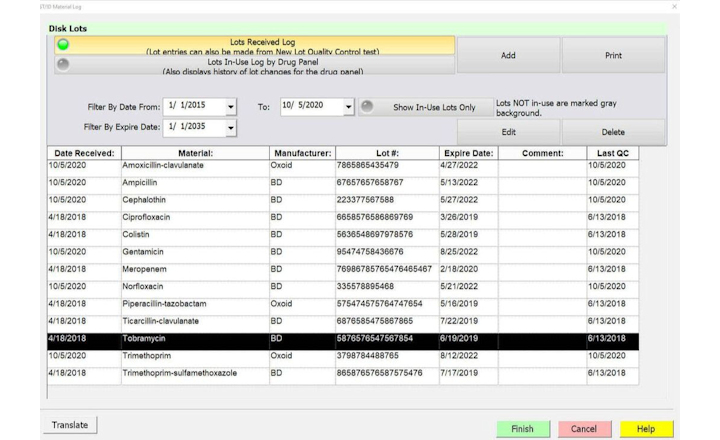
BIOMIC V3's automated QC testing and inventory management features streamline QC and provide electronic documentation of results including:
- Antibiotic Disk QC Testing (CLSI or EUCAST)
- Inventory Management (Disks, Media, Reagents, etc.)
- Reagent QC Testing (Oxidase, Catalase, PYR, etc.)
- Etest/MIC Test Strip QC Testing
- ID Panel QC Testing (API, RapID, Crystal, Liofilchem)
- 96-Well Microtiter QC Testing
- QC Reports with Electronic Signature Review
BIOMIC V3 is designed and manufactured by Giles Scientific in Santa Barbara, California, USA.
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.