EasyDiscTM YEA from IDEXX, used for the enumeration of heterotrophic plate counts (HPC) at 22°C and 36°C in drinking water, has been granted the NF Validation by AFNOR Certification under the reference No 33/09 – 03/22.
The AFNOR Certification follows a study that was carried out by 14 independent expert laboratories. The performance of the EasyDisc method was judged equivalent to the standard method EN ISO 6222, when incubated at 22°C for 68 hours and when incubated at 36°C for 44 hours. Data from the study were then subjected to rigorous scrutiny by experts.
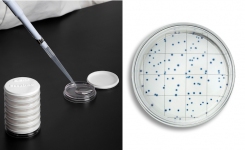
EasyDisc YEA offers an easy solution for HPC testing, with no agar preparation needed, and requires less than one minute of hands-on time to process a sample. Blue colonies reduce ambiguity of results, and colony counting is made easier by the integrated gridlines in the EasyDisc plate.
Proper use of HPC monitoring provides an insight into the effectiveness of ongoing supply chain quality and an indication of potential system failure, while warning of public health hazards.
EasyDisc is available in three different formulations to cover all HPC testing needs: PCA and YEA, which correlate with the pour plate method using plate count agar and yeast extract agar respectively for the testing of drinking and source water; and R2A that correlates with the pour plate method using Reasoner’s 2 agar for the testing of medical and/or pharmaceutical waters.
To learn more about EasyDisc, visit IDEXX or click on the Request Information button below.