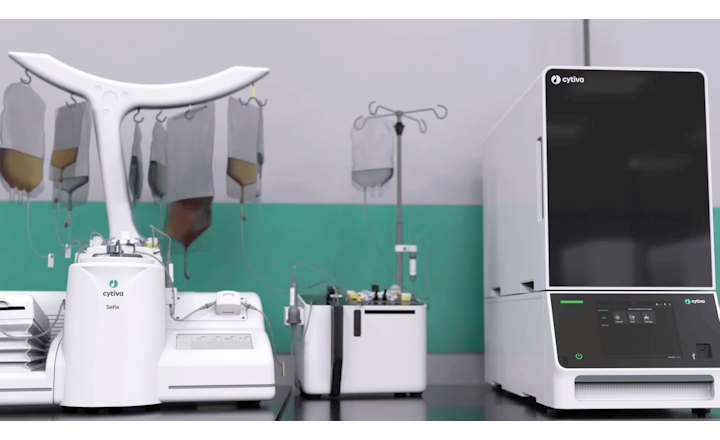
Aseptic fill/finish company Flexicon Liquid Filling, part of Watson-Marlow Fluid Technology Solutions (WMFTS), and barrier technology specialists Franz Ziel GmbH, have launched Cellefill™- a turnkey, vial fill/finish system with an integrated barrier solution.
Enhancing good manufacturing practice (GMP), customer compliance, and efficient aseptic manufacturing is essential as new advanced therapy medicinal products (ATMPs), including cell, gene and biological products advance through clinical trials to commercialisation.
To meet these new requirements, Cellefill integrates the features of Flexicon’s FPC60 aseptic vial filling machine with barrier technologies from Franz Ziel. Cellefill’s process design includes use of asepticsu™ single-use-systems (SUS) and pre-sterilised ready to use (RTU) product containers that enter the Grade A filling zone by a No Touch Transfer (NTT) debagging system. Cellefill is a GMP compliant solution that delivers enhanced levels of process flexibility to accelerate production of ATMPs and biological therapies.
The emerging role of personalised medicine in ATMPs and biological therapies has led to a shift in how manufacturers approach batch production methods. Biopharmaceutical companies are increasingly looking for ways to manufacture the smaller batches needed for patient-specific medicines, in a way that is cost-effective to produce and validate.
Meeting these customer needs, Cellefill maximises production efficiency with features including recipe driven, remote set-up, zero waste start-up and no format parts for the entire vial range.
Steve Adams, Product Manager for Cellefill at WMFTS, says: “Through process flexibility, Cellefill enables the scale out and process repeatability our customers need to assure product quality at all levels of manufacturing. Providing expert guidance and support through a single point of contact, we are a committed project partner to biopharmaceutical companies at the cutting-edge of personalised medicine.
“The challenge for the industry is to produce these new therapies quickly, safely and cost efficiently, with a system that can be rapidly changed over between batch or different small batch medicines.”
Designed to suit various filling requirements and class cleanrooms, the Cellefill solution is available in three models with different barrier technologies. Each model delivers enhanced levels of process and quality assurance with highly accurate peristaltic pump performance, integrated barrier and environmental monitoring.
Cellefill builds on the partners’ experience in providing technology and extensive support through validation, qualification and operator training to facilitate a smooth transition into GMP compliant and cost-effective manufacturing.
Markus Hoersch, division manager sales and marketing from Franz Ziel says: “Cellefill is a fully integrated vial fill/finish and barrier technology solution supporting enhanced levels of product quality and sterility assurance to mitigate risks of contamination. Cellefill adopts pre-designed and configurable modules to reduce project time and fast track therapies to market.
“Cellefill minimises batch changeover time with enhanced cleanability, rapid Vapour-phase Hydrogen Peroxide (VHP) cycle times and gloveless operation in the aseptic core, while retaining the possibility of risk-based compliant interventions if needed in rare incidences to prevent batch loss.”
The Cellefill solution enables a streamlined project timeline with integrated technical and GMP documentation to increase efficiency of the project validation and qualification stages.
The modularity, flexibility, scale out capabilities and efficiencies establish Cellefill as a next generation vial fill/finish solution for new ATMP and biologic products as they advance through clinical trials to commercialisation.
For more information, use the Request Information button below to contact the supplier