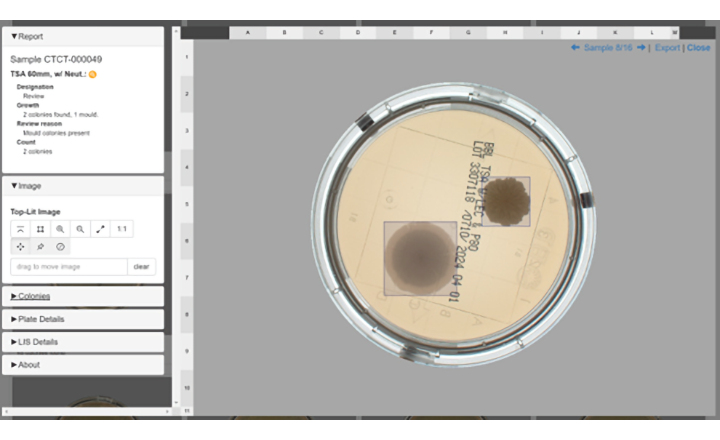
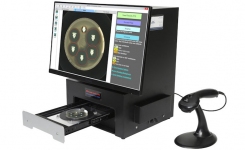
TRINITY V3 utilizes digital imaging to automate zone reading and calculations for antibiotic potency assays. An optional colony counting module is available.
This industrial microbiology system is used primarily in pharmaceutical QA/QC microbiology laboratories.
TRINITY V3 strictly complies with 21 CFR Part 11 electronic signature requirements and follows USP and EP agar diffusion assay methods.
IQ OQ PQ validation documents are included with each system purchase. TRINITY V3 is designed and manufactured by Giles Scientific in California, USA.
Features and benefits include:
- 21 CFR Part 11 compliant
- Follows USP, EP, JP methods
- IQ, OQ, PQ documents included
- No system maintenance
- Instant zone reading & assay calculations
- Customize plate layout and report
- Save high resolution plate images
Video demonstration:
Giles Scientific also sells flat or beveled peni cylinders and peni cylinder dispensers for this assay.
Visit our website here for product information and customer testimonials.