Clever Culture Systems is delighted to announce 5 separate presentations featuring the APAS Independence were presented at the 32nd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) held 23rd - 27th of April 2022 in Lisbon. The clinical data was presented by laboratories from around the world that addressed different aspects of their experience utilising APAS Independence artificial intelligence algorithms.
Results from the studies consistently demonstrated the excellent performance of the APAS Independence artificial intelligence algorithms to interpret microbial growth for a variety of clinical applications. The clinical studies highlight the accuracy of the APAS Independence to effectively screen out negative cultures with a significant reduction in time and the potential to free up clinical laboratory staff to perform more complicated laboratory tasks.
The presentations included the first evaluation of the latest APAS Independence Analysis Module, APAS-AMR for automated antimicrobial susceptibility testing (AST). The study assessed the performance of the APAS-AMR technology for interpretation of disc diffusion AST according to EUCAST, including Rapid-AST applications.
Peter Bradley, General Manager at Clever Culture Systems said: “Laboratories are challenged to do more with the resources they have and that includes staff and equipment. The data on the performance of the artificial intelligence-driven APAS Independence presented at ECCMID 2022 demonstrates the increased utility of the instrument in diverse clinical settings. In addition to screening urine and infection control cultures, the instrument menu is being expanded to interpret disc diffusion for AST determination.”
The 5 Presentations are available below including an interview with the presenting authors of each poster:
- Artificial intelligence (AI)-assisted antimicrobial susceptibility testing (AST): automated imaging and the use of artificial intelligence for interpretation of disc-diffusion AST according to EUCAST, including rapid antimicrobial susceptibility testing (RAST)
- Streamlining Urine Processing with modular automation using DxM Autoplak and APAS Independence: A French experience with CPSE media
- Comparison of the APAS Independence Automated Plate Reader System with manual standard-of-care for processing urine culture specimens
- Evaluation of the APAS Independence with Thermo Fisher Brilliance MRSA Analysis Module at NHS William Harvey Hospital
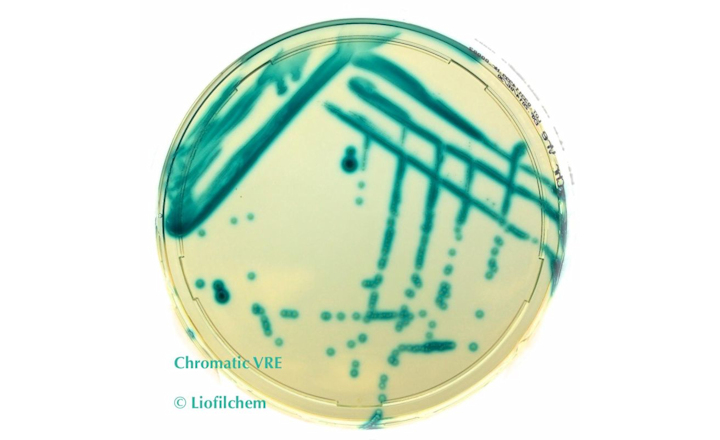
- Artificial intelligence and diagnostic microbiology: comparison of APAS Independence with chromogenic UTI media Analysis Module and routine plate-in-hand with traditional CLED media at NHS William Harvey Hospital
Visit Clever Culture Systems or click on the Request Information button below.