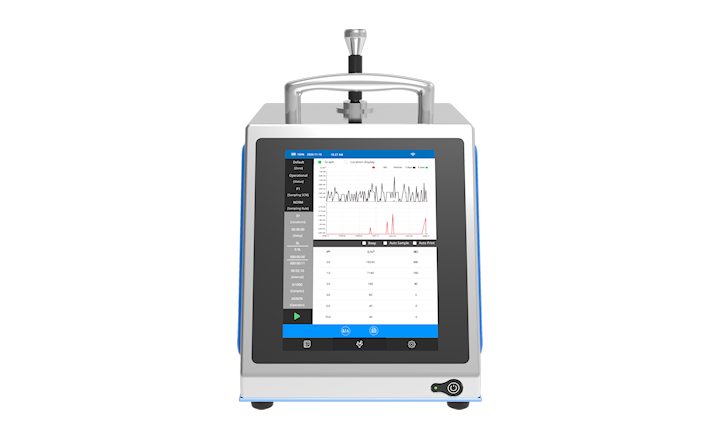
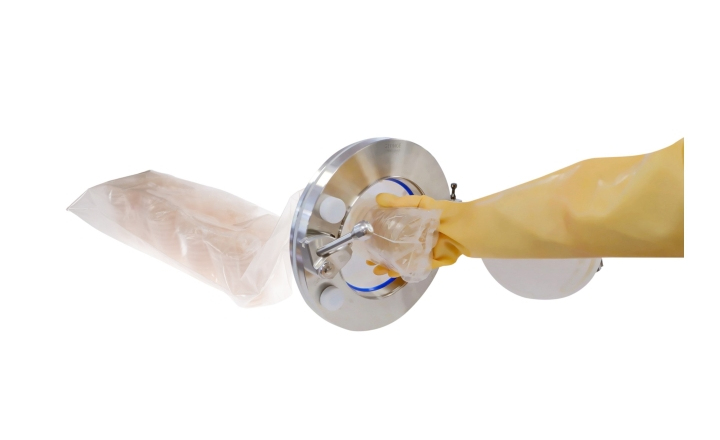
This new book outlines a biocontamination strategy that tracks bio-burden control and reduction at each transition in classified areas of a facility. This key part of controlling risk escalation can lead to the contamination of medicinal products, hence necessary tracking precautions are essential.
Regulatory authorities have challenged pharmaceutical companies, healthcare providers, and those in manufacturing practice to adopt a holistic approach to contamination control. New technologies are needed to introduce barriers between personnel and the environment, and to provide a rapid and more accurate assessment of risk. This book offers guidance on building a complete biocontamination strategy.
Key features of the book are:
- Providing the information necessary for a facility to build a complete biocontamination strategy.
- Helping facilities understand the main biocontamination risks to medicinal products.
- Assisting the reader in navigating regulatory requirements.
- Providing insight into developing an environmental monitoring program.
- Covering the types of rapid microbiological monitoring methods now available, as well as current legislation.
For more details and purchase visit www.elsevier.com/books/biocontamination-control-for-pharmaceuticals-and-healthcare/sandle/978-0-12-814911-9